Vasomotion: cellular background for the oscillator and for the synchronization of smooth muscle cells
- PMID: 15678091
- PMCID: PMC1576043
- DOI: 10.1038/sj.bjp.0706084
Vasomotion: cellular background for the oscillator and for the synchronization of smooth muscle cells
Abstract
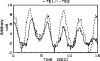
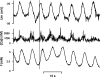
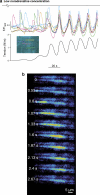
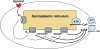
1. Vasomotion is the oscillation of vascular tone with frequencies in the range from 1 to 20 min(-1) seen in most vascular beds. The oscillation originates in the vessel wall and is seen both in vivo and in vitro. 2. Recently, our ideas on the cellular mechanisms responsible for vasomotion have improved. Three different types of cellular oscillations have been suggested. One model has suggested that oscillatory release of Ca2+ from intracellular stores is important (the oscillation is based on a cytosolic oscillator). A second proposed mechanism is an oscillation originating in the sarcolemma (a membrane oscillator). A third mechanism is based on an oscillation of glycolysis (metabolic oscillator). For the two latter mechanisms, only limited experimental evidence is available. 3. To understand vasomotion, it is important to understand how the cells synchronize. For the cytosolic oscillators synchronization may occur via activation of Ca2+-sensitive ion channels by oscillatory Ca2+ release. The ensuing membrane potential oscillation feeds back on the intracellular Ca2+ stores and causes synchronization of the Ca2+ release. While membrane oscillators in adjacent smooth muscle cells could be synchronized through the same mechanism that sets up the oscillation in the individual cells, a mechanism to synchronize the metabolic-based oscillators has not been suggested. 4. The interpretation of the experimental observations is supported by theoretical modelling of smooth muscle cells behaviour, and the new insight into the mechanisms of vasomotion has the potential to provide tools to investigate the physiological role of vasomotion.
Figures


References
-
- AKATA T., KODAMA K., TAKAHASHI S. Role of endothelium in oscillatory contractile responses to various receptor agonists in isolated small mesenteric and epicardial coronary arteries. Jpn. J. Pharmacol. 1995;68:331–343. - PubMed
-
- BAKHRAMOV A., HARTLEY S.A., SALTER K.J., KOZLOWSKI R.Z. Contractile agonists preferentially activate Cl− over K+ currents in arterial myocytes. Biochem. Biophys. Res. Commun. 1996;227:168–175. - PubMed
-
- BENY J.L., CONNAT J.L. An electron-microscopic study of smooth muscle cell dye coupling in the pig coronary arteries. Role of gap junctions. Circ. Res. 1992;70:49–55. - PubMed
-
- BERRIDGE M.J., RAPP P.E. A comparative survey of the function, mechanism and control of cellular oscillators. J. Exp. Biol. 1979;81:217–279. - PubMed
-
- BERTUGLIA S., COLANTUONI A., INTAGLIETTA M. Capillary reperfusion after L-arginine, L-NMMA, and L-NNA treatment in cheek pouch microvasculature. Microvasc. Res. 1995;50:162–174. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

