Kir6.2-dependent high-affinity repaglinide binding to beta-cell K(ATP) channels
- PMID: 15678092
- PMCID: PMC1576033
- DOI: 10.1038/sj.bjp.0706082
Kir6.2-dependent high-affinity repaglinide binding to beta-cell K(ATP) channels
Abstract
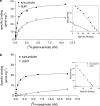
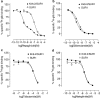
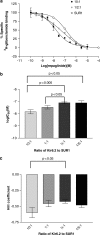
1. The beta-cell K(ATP) channel is composed of two types of subunit - the inward rectifier K(+) channel (Kir6.2) which forms the channel pore, and the sulphonylurea receptor (SUR1), which serves as a regulatory subunit. The N-terminus of Kir6.2 is involved in transduction of sulphonylurea binding into channel closure, and deletion of the N-terminus (Kir6.2DeltaN14) results in functional uncoupling of the two subunits. In this study, we investigate the interaction of the hypoglycaemic agents repaglinide and glibenclamide with SUR1 and the effect of Kir6.2 on this interaction. We further explore how the binding properties of repaglinide and glibenclamide are affected by functional uncoupling of SUR1 and Kir6.2 in Kir6.2DeltaN14/SUR1 channels. All binding experiments are performed on membranes in ATP-free buffer at 37 degrees C. 2. Repaglinide was found to bind with low affinity (K(D)=59+/-16 nM) to SUR1 alone, but with high affinity (increased approximately 150-fold) when SUR1 was co-expressed with Kir6.2 (K(D)=0.42+/-0.03 nM). Glibenclamide, tolbutamide and nateglinide all bound with marginally lower affinity to SUR1 than to Kir6.2/SUR1. 3. Repaglinide bound with low affinity (K(D)=51+/-23 nM) to SUR1 co-expressed with Kir6.2DeltaN14. In contrast, the affinity for glibenclamide, tolbutamide and nateglinide was only mildly changed as compared to wild-type channels. 4. In whole-cell patch-clamp experiments inhibition of Kir6.2DeltaN14/SUR1 currents by both repaglinide and nateglinde is abolished. 5. The results suggest that Kir6.2 causes a conformational change in SUR1 required for high-affinity repaglinide binding, or that the high-affinity repaglinide-binding site includes contributions from both SUR1 and Kir6.2. Glibenclamide, tolbutamide and nateglinide binding appear to involve only SUR1.
Figures

Similar articles
-
Differential interactions of nateglinide and repaglinide on the human beta-cell sulphonylurea receptor 1.Diabetes. 2002 Sep;51(9):2789-95. doi: 10.2337/diabetes.51.9.2789. Diabetes. 2002. PMID: 12196472
-
Involvement of the n-terminus of Kir6.2 in coupling to the sulphonylurea receptor.J Physiol. 1999 Jul 15;518 ( Pt 2)(Pt 2):325-36. doi: 10.1111/j.1469-7793.1999.0325p.x. J Physiol. 1999. PMID: 10381582 Free PMC article.
-
The effects of mitiglinide (KAD-1229), a new anti-diabetic drug, on ATP-sensitive K+ channels and insulin secretion: comparison with the sulfonylureas and nateglinide.Eur J Pharmacol. 2001 Nov 9;431(1):119-25. doi: 10.1016/s0014-2999(01)01412-1. Eur J Pharmacol. 2001. PMID: 11716850
-
Towards selective Kir6.2/SUR1 potassium channel openers, medicinal chemistry and therapeutic perspectives.Curr Med Chem. 2006;13(4):361-76. doi: 10.2174/092986706775527947. Curr Med Chem. 2006. PMID: 16475928 Review.
-
Comparative aspects of the function and mechanism of SUR1 and MDR1 proteins.Biochim Biophys Acta. 1999 Dec 6;1461(2):305-13. doi: 10.1016/s0005-2736(99)00157-1. Biochim Biophys Acta. 1999. PMID: 10581363 Review.
Cited by
-
Individualized therapy for type 2 diabetes: clinical implications of pharmacogenetic data.Mol Diagn Ther. 2012 Oct;16(5):285-302. doi: 10.1007/s40291-012-0002-7. Mol Diagn Ther. 2012. PMID: 23018631 Review.
-
Analysis of two KCNJ11 neonatal diabetes mutations, V59G and V59A, and the analogous KCNJ8 I60G substitution: differences between the channel subtypes formed with SUR1.J Biol Chem. 2009 Mar 13;284(11):6752-62. doi: 10.1074/jbc.M805435200. Epub 2009 Jan 12. J Biol Chem. 2009. PMID: 19139106 Free PMC article.
-
Selectivity of repaglinide and glibenclamide for the pancreatic over the cardiovascular K(ATP) channels.Diabetologia. 2006 Sep;49(9):2039-48. doi: 10.1007/s00125-006-0307-3. Epub 2006 Jul 25. Diabetologia. 2006. PMID: 16865362
-
Importance of the Kir6.2 N-terminus for the interaction of glibenclamide and repaglinide with the pancreatic K(ATP) channel.Naunyn Schmiedebergs Arch Pharmacol. 2012 Mar;385(3):299-311. doi: 10.1007/s00210-011-0709-8. Epub 2011 Nov 15. Naunyn Schmiedebergs Arch Pharmacol. 2012. PMID: 22083559
-
Molecular action of sulphonylureas on KATP channels: a real partnership between drugs and nucleotides.Biochem Soc Trans. 2015 Oct;43(5):901-7. doi: 10.1042/BST20150096. Biochem Soc Trans. 2015. PMID: 26517901 Free PMC article. Review.
References
-
- AGUILAR-BRYAN L., NICHOLS C.G., WECHSLER S.W., CLEMENT J.P., BOYD A.E., GONZLEZ G., HERRERA-SOSA H., NGUY K., BRYAN J., NELSON D.A. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
-
- AMBAVANE V., PATIL R., AINAPURE S.S. Repaglinide: a short acting insulin secretagogue for postprandial hyperglycaemia. J. Postgraduate Med. 2002;48:246–248. - PubMed
-
- ASHCROFT F.M. Ion Channels: exciting times for PIP2. Science. 1998;282:1059–1060. - PubMed
-
- ASHCROFT F.M., GRIBBLE F.M. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. - PubMed
-
- ASHCROFT F.M., RORSMAN P. ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion. Biochem. Soc. trans. 1990;18:109–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

