Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling
- PMID: 15678101
- PMCID: PMC549625
- DOI: 10.1038/sj.emboj.7600569
Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling
Abstract
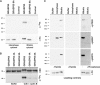
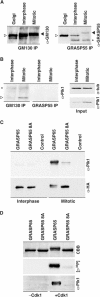
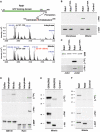
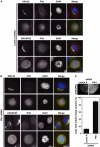
GRASP65, a structural protein of the Golgi apparatus, has been linked to the sensing of Golgi structure and the integration of this information with the control of mitotic entry in the form of a Golgi checkpoint. We show that Cdk1-cyclin B is the major kinase phosphorylating GRASP65 in mitosis, and that phosphorylated GRASP65 interacts with the polo box domain of the polo-like kinase Plk1. GRASP65 is phosphorylated in its C-terminal domain at four consensus sites by Cdk1-cyclin B, and mutation of these residues to alanine essentially abolishes both mitotic phosphorylation and Plk1 binding. Expression of the wild-type GRASP65 C-terminus but not the phosphorylation defective mutant in normal rat kidney cells causes a delay but not the block in mitotic entry expected if this were a true cell cycle checkpoint. These findings identify a Plk1-dependent signalling mechanism potentially linking Golgi structure and cell cycle control, but suggest that this may not be a cell cycle checkpoint in the classical sense.
Figures



References
-
- Acharya U, Mallabiabarrena A, Acharya JK, Malhotra V (1998) Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell 92: 183–192 - PubMed
-
- Allan BB, Moyer BD, Balch WE (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289: 444–448 - PubMed
-
- Bailly E, McCaffrey M, Touchot N, Zahraoui A, Goud B, Bornens M (1991) Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature 350: 715–718 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

