Redefining the subcellular location and transport of APC: new insights using a panel of antibodies
- PMID: 15678162
- PMCID: PMC1299239
- DOI: 10.1038/sj.embor.7400329
Redefining the subcellular location and transport of APC: new insights using a panel of antibodies
Abstract
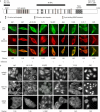
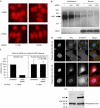
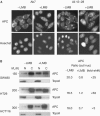
Adenomatous polyposis coli (APC) is a tumour suppressor involved in colon cancer progression. We and others previously described nuclear-cytoplasmic shuttling of APC. However, there are conflicting reports concerning the localization of endogenous wild-type and tumour-associated, truncated APC. To resolve this issue, we compared APC localization using immunofluorescence (IF) microscopy and cell fractionation with nine different APC antibodies. We found that three commonly used APC antibodies showed nonspecific nuclear staining by IF and validated this conclusion in cells where APC was inactivated using small interfering RNA or Cre/Flox. Fractionation showed that wild-type and truncated APC from colon cancer cells were primarily cytoplasmic, but increased in the nucleus after leptomycin B treatment, consistent with CRM1-dependent nuclear export. In contrast to recent reports, our biochemical data indicate that APC nuclear localization is not regulated by changes in cell density, and that APC nuclear export is not prevented by truncating mutations in cancer. These results verify that the bulk of APC resides in the cytoplasm and indicate the need for caution when evaluating the nuclear accumulation of APC.
Figures

Similar articles
-
Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover.Nat Cell Biol. 2000 Sep;2(9):653-60. doi: 10.1038/35023605. Nat Cell Biol. 2000. PMID: 10980707
-
Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm.Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):12085-90. doi: 10.1073/pnas.220401797. Proc Natl Acad Sci U S A. 2000. PMID: 11035805 Free PMC article.
-
ARM domain-dependent nuclear import of adenomatous polyposis coli protein is stimulated by the B56 alpha subunit of protein phosphatase 2A.J Biol Chem. 2001 Dec 7;276(49):45833-9. doi: 10.1074/jbc.M107149200. Epub 2001 Oct 3. J Biol Chem. 2001. PMID: 11585828
-
The adenomatous polyposis coli (APC) tumour suppressor--genetics, function and disease.Mol Med Today. 2000 Dec;6(12):462-9. doi: 10.1016/s1357-4310(00)01828-1. Mol Med Today. 2000. PMID: 11099951 Review.
-
Adenomatous polyposis coli (Apc) tumor suppressor gene as a multifunctional gene.Anat Sci Int. 2005 Sep;80(3):121-31. doi: 10.1111/j.1447-073x.2005.00106.x. Anat Sci Int. 2005. PMID: 16158975 Review.
Cited by
-
Validation and application of a novel APC antibody in western blotting, immunoprecipitation, and immunohistochemistry.Med Mol Morphol. 2018 Dec;51(4):227-236. doi: 10.1007/s00795-018-0196-9. Epub 2018 Jun 19. Med Mol Morphol. 2018. PMID: 29923125
-
Actin nucleators in the nucleus: an emerging theme.J Cell Sci. 2012 Aug 1;125(Pt 15):3519-27. doi: 10.1242/jcs.099523. Epub 2012 Aug 30. J Cell Sci. 2012. PMID: 22935654 Free PMC article. Review.
-
Immunopurification of adenomatous polyposis coli (APC) proteins.BMC Res Notes. 2013 Oct 25;6:429. doi: 10.1186/1756-0500-6-429. BMC Res Notes. 2013. PMID: 24156781 Free PMC article.
-
Adenomatous polyposis coli regulates axon arborization and cytoskeleton organization via its N-terminus.PLoS One. 2011;6(9):e24335. doi: 10.1371/journal.pone.0024335. Epub 2011 Sep 6. PLoS One. 2011. PMID: 21915313 Free PMC article.
-
EB1 is required for spindle symmetry in mammalian mitosis.PLoS One. 2011;6(12):e28884. doi: 10.1371/journal.pone.0028884. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22216133 Free PMC article.
References
-
- Brocardo MG et al. (2001) APC senses cell–cell contacts and moves to the nucleus upon their disruption. Biochem Biophys Res Commun 284: 982–986 - PubMed
-
- Davies ML, Roberts GT, Spiller DG, Wakeman JA (2004) Density-dependent location and interactions of truncated APC and β-catenin. Oncogene 23: 1412–1419 - PubMed
-
- Dikovskaya D, Zumbrunn J, Penman GA, Näthke IS (2001) The adenomatous polyposis coli protein: in the limelight out at the edge. Trends Cell Biol 11: 378–384 - PubMed
-
- Erdman KS, Kuhlmann J, Lessmann V, Herrman L, Eulenburg V, Muller O, Heumann R (2000) The adenomatous polyposis coli-protein interacts with the protein tyrosine phosphatase PTP-BL via an alternatively spliced PDZ domain. Oncogene 19: 3894–3901 - PubMed
-
- Fagman H et al. (2003) Nuclear accumulation of full-length and truncated adenomatous polyposis coli protein in tumour cells depends on proliferation. Oncogene 22: 6013–6022 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

