Secondary neurons are arrested in an immature state by formation of epithelial vesicles during neurogenesis of the spider Cupiennius salei
- PMID: 15679931
- PMCID: PMC544935
- DOI: 10.1186/1742-9994-1-3
Secondary neurons are arrested in an immature state by formation of epithelial vesicles during neurogenesis of the spider Cupiennius salei
Abstract
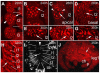
BACKGROUND: In the spider Cupiennius salei about 30 groups of neural precursors are generated per hemi-segment during early neurogenesis. Analysis of the ventral neuromeres after invagination of the primary neural precursor groups revealed that secondary neural precursors arise during late embryogenesis that partially do not differentiate until larval stages. RESULTS: In contrast to the primary groups, the secondary invaginating cells do not detach from each other after invagination but maintain their epithelial character and form so-called epithelial vesicles. As revealed by dye labeling, secondary neural precursors within epithelial vesicles do not show any morphological features of differentiation indicating that the formation of epithelial vesicles after invagination leads to a delay in the differentiation of the corresponding neural precursors. About half of the secondary neural precursor groups do not dissociate from each other during embryogenesis indicating that they provide neural precursors for larval and adult stages. CONCLUSIONS: Secondary neural precursors are arrested in an immature state by formation of epithelial vesicles. This mechanism facilitates the production of larval neural precursors during embryogenesis. I discuss the evolutionary changes that have occured during neural precursor formation in the arthropod group and present a model for the basal mode of neurogenesis.
Figures



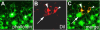


Similar articles
-
Neurogenesis in the spider: new insights from comparative analysis of morphological processes and gene expression patterns.Arthropod Struct Dev. 2003 Aug;32(1):5-16. doi: 10.1016/S1467-8039(03)00041-0. Arthropod Struct Dev. 2003. PMID: 18088993
-
The role of Notch signalling and numb function in mechanosensory organ formation in the spider Cupiennius salei.Dev Biol. 2009 Mar 1;327(1):121-31. doi: 10.1016/j.ydbio.2008.12.004. Epub 2008 Dec 16. Dev Biol. 2009. PMID: 19121304
-
Evolutionary changes in sensory precursor formation in arthropods: embryonic development of leg sensilla in the spider Cupiennius salei.Dev Biol. 2008 Jan 15;313(2):659-73. doi: 10.1016/j.ydbio.2007.11.003. Epub 2007 Nov 17. Dev Biol. 2008. PMID: 18054903
-
Cupiennius salei and Achaearanea tepidariorum: Spider models for investigating evolution and development.Bioessays. 2008 May;30(5):487-98. doi: 10.1002/bies.20744. Bioessays. 2008. PMID: 18404731 Review.
-
"Arrested development". Immature, but not recently generated, neurons in the adult brain.Arch Ital Biol. 2010 Jun;148(2):159-72. Arch Ital Biol. 2010. PMID: 20830977 Review.
Cited by
-
Velvet worm development links myriapods with chelicerates.Proc Biol Sci. 2009 Oct 22;276(1673):3571-9. doi: 10.1098/rspb.2009.0950. Epub 2009 Jul 29. Proc Biol Sci. 2009. PMID: 19640885 Free PMC article.
-
The evolution of early neurogenesis.Dev Cell. 2015 Feb 23;32(4):390-407. doi: 10.1016/j.devcel.2015.02.004. Dev Cell. 2015. PMID: 25710527 Free PMC article. Review.
-
Embryonic neurogenesis in Pseudopallene sp. (Arthropoda, Pycnogonida) includes two subsequent phases with similarities to different arthropod groups.Evodevo. 2013 Nov 29;4(1):32. doi: 10.1186/2041-9139-4-32. Evodevo. 2013. PMID: 24289241 Free PMC article.
-
The 'ventral organs' of Pycnogonida (Arthropoda) are neurogenic niches of late embryonic and post-embryonic nervous system development.PLoS One. 2014 Apr 15;9(4):e95435. doi: 10.1371/journal.pone.0095435. eCollection 2014. PLoS One. 2014. PMID: 24736377 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

