Persistent borna disease virus infection confers instability of HSP70 mRNA in glial cells during heat stress
- PMID: 15681405
- PMCID: PMC546570
- DOI: 10.1128/JVI.79.4.2033-2041.2005
Persistent borna disease virus infection confers instability of HSP70 mRNA in glial cells during heat stress
Abstract
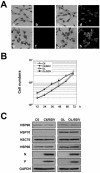
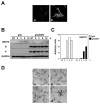
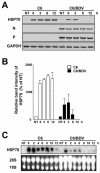
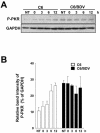
Borna disease virus (BDV) is a highly neurotropic RNA virus that causes neurological disorders in many vertebrate species. Although BDV readily establishes lasting persistence, persistently infected cells maintain an apparently normal cell phenotype in terms of morphology, viability, and proliferation. In this study, to understand the regulation of stress responses in BDV infection, we investigated the expression of heat shock proteins (HSPs) in glial cells persistently infected with BDV. Interestingly, we found that BDV persistence did not upregulate HSP70 expression even in cells treated with heat stress. Furthermore, BDV-infected glial cells exhibited rapid rounding and detachment from the culture plate under various stressful conditions. Immunofluorescence analysis demonstrated that heat stress rapidly disrupts the cell cytoskeleton only in persistently infected cells, suggesting a lack of thermotolerance. Intriguingly, we found that although persistently infected glial cells expressed HSP70 mRNA after heat stress, its expression rapidly disappeared during the recovery period. These observations indicated that persistent BDV infection may affect the stability of HSP70 mRNA. Finally, we found that the double-stranded RNA-dependent protein kinase (PKR) is expressed at a constant level in persistently infected cells with or without heat shock. Considering the interrelationship between HSP70 and PKR production, our data suggest that BDV infection disturbs the cellular stress responses to abolish antiviral activities and maintain persistence.
Figures


Similar articles
-
Restricted expression of Borna disease virus glycoprotein in brains of experimentally infected Lewis rats.Neuropathol Appl Neurobiol. 2008 Dec;34(6):590-602. doi: 10.1111/j.1365-2990.2008.00940.x. Epub 2008 Feb 14. Neuropathol Appl Neurobiol. 2008. PMID: 18282160
-
Layer specific changes of astroglial gap junctions in the rat cerebellar cortex by persistent Borna Disease Virus infection.Brain Res. 2008 Jul 11;1219:143-58. doi: 10.1016/j.brainres.2008.04.062. Epub 2008 Apr 30. Brain Res. 2008. PMID: 18538309
-
Downregulation of an astrocyte-derived inflammatory protein, S100B, reduces vascular inflammatory responses in brains persistently infected with Borna disease virus.J Virol. 2007 Jun;81(11):5940-8. doi: 10.1128/JVI.02137-06. Epub 2007 Mar 21. J Virol. 2007. PMID: 17376896 Free PMC article.
-
Novel insights into the regulation of the viral polymerase complex of neurotropic Borna disease virus.Virus Res. 2005 Aug;111(2):148-60. doi: 10.1016/j.virusres.2005.04.006. Virus Res. 2005. PMID: 15992626 Review.
-
Bornavirus and the brain.J Infect Dis. 2002 Dec 1;186 Suppl 2:S241-7. doi: 10.1086/344936. J Infect Dis. 2002. PMID: 12424704 Review.
Cited by
-
Conserved microRNA mediates heating tolerance in germ cells versus surrounding somatic cells.RNA Biol. 2019 Oct;16(10):1494-1503. doi: 10.1080/15476286.2019.1639311. Epub 2019 Jul 15. RNA Biol. 2019. PMID: 31276432 Free PMC article.
-
Antigenic diversification is correlated with increased thermostability in a mammalian virus.Virology. 2016 Sep;496:203-214. doi: 10.1016/j.virol.2016.06.009. Epub 2016 Jun 23. Virology. 2016. PMID: 27344137 Free PMC article.
-
Borna disease virus infects human neural progenitor cells and impairs neurogenesis.J Virol. 2012 Mar;86(5):2512-22. doi: 10.1128/JVI.05663-11. Epub 2011 Dec 21. J Virol. 2012. PMID: 22190725 Free PMC article.
-
Susceptibility of human and rat neural cell lines to infection by SARS-coronavirus.Biochem Biophys Res Commun. 2005 Aug 19;334(1):79-85. doi: 10.1016/j.bbrc.2005.06.061. Biochem Biophys Res Commun. 2005. PMID: 15992768 Free PMC article.
References
-
- Asea, A., S. K. Kraeft, E. A. Kurt-Jones, M. A. Stevenson, L. B. Chen, R. W. Finberg, G. C. Koo, and S. K. Calderwood. 2000. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 6:435-442. - PubMed
-
- Bechtold, D. A., S. J. Rush, and I. R. Brown. 2000. Localization of the heat-shock protein Hsp70 to the synapse following hyperthermic stress in the brain. J. Neurochem. 74:641-646. - PubMed
-
- Beck, J., and M. Nassal. 2003. Efficient Hsp90-independent in vitro activation by Hsc70 and Hsp40 of duck hepatitis B virus reverse transcriptase, an assumed Hsp90 client protein. J. Biol. Chem. 278:36128-36138. - PubMed
-
- Bodega, G., C. Hernandez, I. Suarez, M. Martin, and B. Fernandez. 2002. HSP70 constitutive expression in rat central nervous system from postnatal development to maturity. J. Histochem. Cytochem. 50:1161-1168. - PubMed
-
- Chawla-Sarkar, M., D. J. Lindner, Y. F. Liu, B. R. Williams, G. C. Sen, R. H. Silverman, and E. C. Borden. 2003. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 8:237-249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

