Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection
- PMID: 15681407
- PMCID: PMC546549
- DOI: 10.1128/JVI.79.4.2050-2057.2005
Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection
Abstract
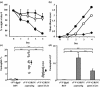
Respiratory syncytial virus (RSV) is a major viral pathogen of infants and the elderly. Significant morbidity is caused by an overexuberant mixed lung cell infiltrate, which is thought to be driven by chemokines. One of the main chemotactic mediators responsible for the movement of eosinophils is CCL11 (eotaxin). Using a mouse model of eosinophilic bronchiolitis induced by RSV, we show here that treatment in vivo with a blocking antibody to CCL11 greatly reduces lung eosinophilia and disease severity. In addition, anti-CCL11 caused a striking inhibition of CD4-T-cell influx and shifted cytokine production away from interleukin-5 without reducing the resistance to viral replication. These results suggest that in addition to influencing eosinophil diapedesis and survival, anti-CCL11 has an action on T cells. These studies strengthen the case for anti-CCL11 treatment of Th2-driven diseases.
Figures




References
-
- Chensue, S. W., N. W. Lukacs, T. Y. Yang, X. Shang, K. A. Frait, S. L. Kunkel, T. Kung, M. T. Wiekowski, J. A. Hedrick, D. N. Cook, A. Zingoni, S. K. Narula, A. Zlotnik, F. J. Barrat, A. O'Garra, M. Napolitano, and S. A. Lira. 2001. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J. Exp. Med. 193:573-584. - PMC - PubMed
-
- D'Ambrosio, D., A. Iellem, R. Bonecchi, D. Mazzeo, S. Sozzani, A. Mantovani, and F. Sinigaglia. 1998. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J. Immunol. 161:5111-5115. - PubMed
-
- de Lavareille, A., F. Roufosse, L. Schandene, P. Stordeur, E. Cogan, and M. Goldman. 2001. Clonal Th2 cells associated with chronic hypereosinophilia: TARC-induced CCR4 down-regulation in vivo. Eur. J. Immunol. 31:1037-1046. - PubMed
-
- Fujisawa, T., Y. Kato, J. Atsuta, A. Terada, K. Iguchi, H. Kamiya, H. Yamada, T. Nakajima, M. Miyamasu, and K. Hirai. 2000. Chemokine production by the BEAS-2B human bronchial epithelial cells: differential regulation of eotaxin, IL-8, and RANTES by TH2- and TH1-derived cytokines. J. Allergy Clin. Immunol. 105:126-133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

