Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity
- PMID: 15681453
- PMCID: PMC546590
- DOI: 10.1128/JVI.79.4.2528-2540.2005
Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity
Abstract
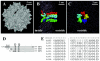
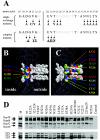
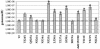
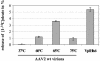
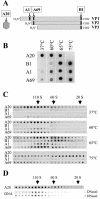
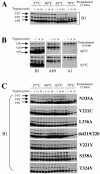
Adeno-associated virus type 2 (AAV2) capsids show 12 pores at the fivefold axes of symmetry. We mutated amino acids which constitute these pores to investigate possible functions of these structures within the AAV2 life cycle. Mutants with alterations in conserved residues were impaired mainly in genome packaging or infectivity, whereas few mutants were affected in capsid assembly. The packaging phenotype was characterized by increased capsid-per-genome ratios. Analysis of capsid-associated DNA versus encapsidated DNA revealed that this observation was due to reduced and not partial DNA encapsidation. Most mutants with impaired infectivity showed a decreased capability to expose their VP1 N termini. As a consequence, the activation of phospholipase A2 (PLA2) activity, which is essential for efficient infection, was affected on intact capsids. In a few mutants, the exposure of VP1 N termini and the development of PLA2 activity were associated with enhanced capsid instability, which is obviously also deleterious for virus infection. Therefore, PLA2 activity seems to be required on intact capsids for efficient infection. In conclusion, these results suggest that the pores at the fivefold axes function not only as portals for AAV2 single-stranded DNA packaging but also as channels for presentation of the PLA2 domain on AAV2 virions during infection.
Figures


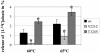
References
-
- Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. - PubMed
-
- Agbandje, M., S. Kajigaya, R. McKenna, N. S. Young, and M. G. Rossmann. 1994. The structure of human parvovirus B19 at 8 A resolution. Virology 203:106-115. - PubMed
-
- Agbandje-McKenna, M., A. L. Llamas-Saiz, F. Wang, P. Tattersall, and M. G. Rossmann. 1998. Functional implications of the structure of the murine parvovirus, minute virus of mice. Structure 6:1369-1381. - PubMed
-
- Atchison, R. W., B. C. Casto, and W. M. Hammon. 1965. Adeno-associated defective virus particles. Science 149:754-756. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

