Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition
- PMID: 15681824
- PMCID: PMC1602317
- DOI: 10.1016/S0002-9440(10)62263-8
Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition
Abstract
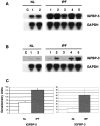
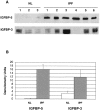
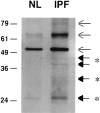
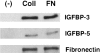
Idiopathic pulmonary fibrosis (IPF) is a fibrotic disease of unknown etiology that results in significant morbidity and mortality. The pathogenesis of IPF is not completely understood. Because recent studies have implicated insulin-like growth factor-I (IGF-I) in the pathogenesis of fibrosis, we examined the expression and function of insulin-like growth factor binding proteins (IGFBP)-3 and -5 in IPF. IGFBP-3 and -5 levels were increased in vivo in IPF lung tissues and in vitro in fibroblasts cultured from IPF lung. The IGFBPs secreted by IPF fibroblasts are functionally active and can bind IGF-I, and IGFBPs secreted by primary fibroblasts bind extracellular matrix components. Our results also suggest that IGFBPs may be involved in the initiation and/or perpetuation of fibrosis by virtue of their ability to induce the production of extracellular matrix components such as collagen type I and fibronectin in normal primary adult lung fibroblasts. Although transforming growth factor-beta increased IGFBP-3 production by primary fibroblasts in a time-dependent manner, IGFBP-5 levels were not increased by transforming growth factor-beta. Taken together, our results suggest that IGFBPs play an important role in the development of fibrosis in IPF.
Figures





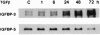
Similar articles
-
NADPH oxidase-mediated induction of reactive oxygen species and extracellular matrix deposition by insulin-like growth factor binding protein-5.Am J Physiol Lung Cell Mol Physiol. 2019 Apr 1;316(4):L644-L655. doi: 10.1152/ajplung.00106.2018. Epub 2019 Feb 27. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30810066 Free PMC article.
-
Testosterone and insulin-like growth factor (IGF) I interact in controlling IGF-binding protein production in androgen-responsive foreskin fibroblasts.J Clin Endocrinol Metab. 2000 Apr;85(4):1627-33. doi: 10.1210/jcem.85.4.6517. J Clin Endocrinol Metab. 2000. PMID: 10770208
-
Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltration.Am J Pathol. 2006 Nov;169(5):1633-42. doi: 10.2353/ajpath.2006.060501. Am J Pathol. 2006. PMID: 17071587 Free PMC article.
-
Abnormal expression of IGF-binding proteins, an initiating event in idiopathic pulmonary fibrosis?Pathol Res Pract. 2010 Aug 15;206(8):537-43. doi: 10.1016/j.prp.2010.03.010. Epub 2010 May 7. Pathol Res Pract. 2010. PMID: 20452131 Review.
-
Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts.Lung. 2005 Jul-Aug;183(4):225-37. doi: 10.1007/s00408-004-2534-z. Lung. 2005. PMID: 16211459 Review.
Cited by
-
Pathogenesis of pulmonary fibrosis in systemic sclerosis: lessons from interstitial lung disease.Curr Rheumatol Rep. 2010 Feb;12(1):19-25. doi: 10.1007/s11926-009-0071-8. Curr Rheumatol Rep. 2010. PMID: 20425529 Review.
-
Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis.Cell Mol Life Sci. 2021 Mar;78(5):2031-2057. doi: 10.1007/s00018-020-03693-7. Epub 2020 Nov 17. Cell Mol Life Sci. 2021. PMID: 33201251 Free PMC article. Review.
-
Targeting insulin-like growth factor-I and insulin-like growth factor-binding protein-3 signaling pathways. A novel therapeutic approach for asthma.Am J Respir Cell Mol Biol. 2014 Apr;50(4):667-77. doi: 10.1165/rcmb.2013-0397TR. Am J Respir Cell Mol Biol. 2014. PMID: 24219511 Free PMC article. Review.
-
The IGF System and Aging.Endocr Rev. 2025 Mar 11;46(2):214-223. doi: 10.1210/endrev/bnae029. Endocr Rev. 2025. PMID: 39418083 Free PMC article. Review.
-
The K+ channel KCa3.1 as a novel target for idiopathic pulmonary fibrosis.PLoS One. 2013 Dec 31;8(12):e85244. doi: 10.1371/journal.pone.0085244. eCollection 2013. PLoS One. 2013. PMID: 24392001 Free PMC article.
References
-
- King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:1171–1181. - PubMed
-
- King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis. Relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. - PubMed
-
- Selman M, King T, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001. 2001;134:136–151. - PubMed
-
- Ward A, Hunninghake GW. Lung Inflammation and fibrosis. Am J Respir Crit Care Med. 1998;157:S123–S129. - PubMed
-
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine Rev. 1995;16:3–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

