Isolation of renal progenitor cells from adult human kidney
- PMID: 15681837
- PMCID: PMC1602314
- DOI: 10.1016/S0002-9440(10)62276-6
Isolation of renal progenitor cells from adult human kidney
Abstract
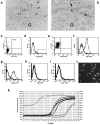
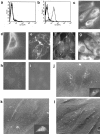
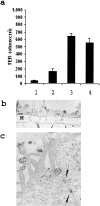
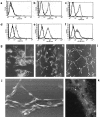
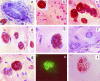
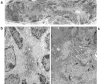
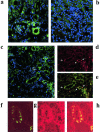
We describe here isolation and characterization of CD133+ cells derived from normal adult human kidney. These cells lacked the expression of hematopoietic markers and expressed PAX-2, an embryonic renal marker, suggesting their renal origin. Renal tissue-derived CD133+ cells and clones of individual cells were capable of expansion and limited self-renewal and differentiated in vitro into epithelial or endothelial cells. On subcutaneous implantation in SCID mice, the undifferentiated cells formed tubular structures expressing renal epithelial markers. At variance, when differentiated in endothelial cells, these cells formed functional vessels. On intravenous injection in SCID mice with glycerol-induced tubulonecrosis, the in vitro expanded renal-derived CD133+ cells homed into the injured kidney and integrated in tubules. We propose that CD133+ cells from kidney represent a multipotent adult resident stem cell population that may contribute to the repair of renal injury.
Figures


References
-
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. - PubMed
-
- Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. - PubMed
-
- Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int. 2002;61:387–395. - PubMed
-
- Oliver JA, Barasch J, Yang J, Herzlinger D, Al-Awqati Q. Metanephric mesenchyme contains embryonic renal stem cells. Am J Physiol. 2002;283:F799–F809. - PubMed
-
- Robert B, St. John PL, Abrahamson DR. Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am J Physiol. 1998;275:F164–F172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

