Receptor-mediated tobacco toxicity: regulation of gene expression through alpha3beta2 nicotinic receptor in oral epithelial cells
- PMID: 15681842
- PMCID: PMC1602318
- DOI: 10.1016/s0002-9440(10)62281-x
Receptor-mediated tobacco toxicity: regulation of gene expression through alpha3beta2 nicotinic receptor in oral epithelial cells
Abstract
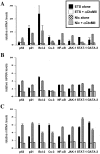
Tobacco is a known cause of oral disease but the mechanism remains elusive. Nicotine (Nic) is a likely culprit of pathobiological effects because it displaces the local cytotransmitter acetylcholine from the nicotinic receptors (nAChRs) expressed by oral keratinocytes (KCs). To gain a mechanistic insight into tobacco-induced morbidity in the oral cavity, we studied effects of exposures to environmental tobacco smoke (ETS) versus equivalent concentration of pure Nic on human and murine KCs. Both ETS and Nic up-regulated expression of cell cycle and apoptosis regulators, differentiation marker filaggrin, and signal transduction factors at both the mRNA and protein levels. These changes could be abolished in cultured human oral KCs transfected with anti-alpha3 small interfering RNA or treated with the alpha3beta2-preferring antagonist alpha-conotoxin MII. Functional inactivation of alpha3-mediated signaling in alpha3-/- mutant KCs prevented most of the ETS/Nic-dependent changes in gene expression. To determine relevance of the in vitro findings to the in vivo situation, we studied gene expression in oral mucosa of neonatal alpha3+/+ and alpha3-/- littermates delivered by heterozygous mice soon after their exposures to ETS or equivalent concentration of pure Nic in drinking water. In addition to reverse transcriptase-polymerase chain reaction and Western blot, the ETS/Nic-dependent alterations in gene expression were also detected by semiquantitative immunofluorescence assay directly in KCs comprising murine oral mucosa. Only wild-type mice consistently developed significant (P < 0.05) changes in the gene expression. These results identified alpha3beta2 nAChR as a major receptor mediating effects of tobacco products on KC gene expression. Real-time polymerase chain reaction demonstrated that in all three model systems the common genes targeted by alpha3beta2-mediated ETS/Nic toxicity were p21, Bcl-2, NF-kappaB, and STAT-1. The expression of the nAChR subunits alpha5 and beta2 and the muscarinic receptor subtypes M(2) and M(3) was also altered. This novel mechanism offers innovative solutions to ameliorate the tobacco-related cell damage and intercede in disease pathways, and may shed light on general mechanisms regulating and driving tobacco-related morbidity in human cells.
Figures
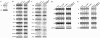

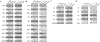
References
-
- Calsina G, Ramon JM, Echeverria JJ. Effects of smoking on periodontal tissues. J Clin Periodontol. 2002;29:771–776. - PubMed
-
- Palmer RM, Scott DA, Meekin TN, Poston RN, Odell EW, Wilson RF. Potential mechanisms of susceptibility to periodontitis in tobacco smokers. J Periodontal Res. 1999;34:363–369. - PubMed
-
- Taybos G. Oral changes associated with tobacco use. Am J Med Sci. 2003;326:179–182. - PubMed
-
- Obeid P, Bercy P. Effects of smoking on periodontal health: a review. Adv Ther. 2000;17:230–237. - PubMed
-
- Chowdhury P, MacLeod S, Udupa KB, Rayford PL. Pathophysiological effects of nicotine on the pancreas: an update. Exp Biol Med (Maywood) 2002;227:445–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

