Platelet-derived growth factor modulates rat vascular smooth muscle cell responses on laminin-5 via mitogen-activated protein kinase-sensitive pathways
- PMID: 15683539
- PMCID: PMC552332
- DOI: 10.1186/1478-811X-3-2
Platelet-derived growth factor modulates rat vascular smooth muscle cell responses on laminin-5 via mitogen-activated protein kinase-sensitive pathways
Abstract
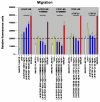
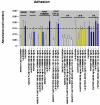
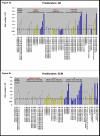
BACKGROUND: A treatment to remove vascular blockages, angioplasty, can cause damage to the vessel wall and a subsequent abnormal wound healing response, known as restenosis. Vascular smooth muscle cells (VSMC) lining the vessel wall respond to growth factors and other stimuli released by injured cells. However, the extracellular matrix (ECM) may differentially modulate VSMC responses to these growth factors, such as proliferation, migration and adhesion. Our previous reports of low-level expression of one ECM molecule, laminin-5, in normal and injured vessels suggest that laminin-5, in addition to growth factors, may mediate VSMC response following vascular injury. To elucidate VSMC response on laminin-5 we investigated-the role of platelet-derived growth factor (PDGF-BB) in activating the mitogen-activated protein kinase (MAPK) signaling cascade as a possible link between growth-factor initiated phenotypic changes in vitro and the ECM. RESULTS: Using a system of in vitro assays we assessed rat vascular smooth muscle cell (rVSMC) responses plated on laminin-5 to the addition of exogenous, soluble PDGF-BB. Our results indicate that although laminin-5 induces haptotactic migration of rVSMC, the addition of PDGF-BB significantly increases rVSMC migration on laminin-5, which is inhibited in a dose-dependent manner by the MAPK inhibitor, PD98059, and transforming growth factor (TGF-beta1). In addition, PDGF-BB greatly reduces rVSMC adhesion to laminin-5, an effect that is reversible by MAPK inhibition or the addition of TGF-beta1. In addition, this reduction in adhesion is less significant on another ECM substrate, fibronectin and is reversible using TGF-beta1 but not MAPK inhibition. PDGF-BB also strongly increased rVSMC proliferation on laminin-5, but had no effect on rVSMC plated on fibronectin. Finally, plating rVSMC on laminin-5 did not induce an increase in MAPK activation, while plating on fibronectin or the addition of soluble PDGF-BB did. CONCLUSION: These results suggest that rVSMC binding to laminin-5 activates integrin-dependent intracellular signaling cascades that are different from those of fibronectin or PDGF-BB, causing rVSMC to respond more acutely to the inhibition of MAPK. In contrast, our results suggest that fibronectin and PDGF-BB may activate parallel, reinforcing intracellular signaling cascades that converge in the activation of MAPK and are therefore less sensitive to MAPK inhibition. These results suggest a partial mechanism to explain the regulation of rVSMC behaviors, including migration, adhesion, and proliferation that may be responsible for the progression of restenosis.
Figures


Similar articles
-
ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5.Biochem Biophys Res Commun. 2002 May 10;293(3):1000-6. doi: 10.1016/S0006-291X(02)00331-5. Biochem Biophys Res Commun. 2002. PMID: 12051759
-
Platelet-derived growth factor-BB, insulin-like growth factor-I, and phorbol ester activate different signaling pathways for stimulation of vascular smooth muscle cell migration.Exp Cell Res. 1998 Aug 1;242(2):548-60. doi: 10.1006/excr.1998.4138. Exp Cell Res. 1998. PMID: 9683541
-
Low-power laser irradiation inhibits PDGF-BB-induced migration and proliferation via apoptotic cell death in vascular smooth muscle cells.Lasers Med Sci. 2017 Dec;32(9):2121-2127. doi: 10.1007/s10103-017-2338-z. Epub 2017 Oct 5. Lasers Med Sci. 2017. PMID: 28983687
-
Mitogen-activated protein kinase activation is involved in platelet-derived growth factor-directed migration by vascular smooth muscle cells.Hypertension. 1997 Jan;29(1 Pt 2):334-9. doi: 10.1161/01.hyp.29.1.334. Hypertension. 1997. PMID: 9039124
-
Differential effects of intravenous anesthetics on PDGF-BB-induced vascular smooth muscle cell migration.Cell Physiol Biochem. 2014;33(6):1827-37. doi: 10.1159/000362961. Epub 2014 Jun 20. Cell Physiol Biochem. 2014. PMID: 24969327
Cited by
-
Hyperactive RAS/PI3-K/MAPK Signaling Cascade in Migration and Adhesion of Nf1 Haploinsufficient Mesenchymal Stem/Progenitor Cells.Int J Mol Sci. 2015 Jun 1;16(6):12345-59. doi: 10.3390/ijms160612345. Int J Mol Sci. 2015. PMID: 26039236 Free PMC article.
-
Cubeben induces autophagy via PI3K-AKT-mTOR pathway to protect primary neurons against amyloid beta in Alzheimer's disease.Cytotechnology. 2019 Jun;71(3):679-686. doi: 10.1007/s10616-019-00313-6. Epub 2019 Apr 9. Cytotechnology. 2019. PMID: 30968233 Free PMC article.
-
Dissection of the osteogenic effects of laminin-332 utilizing specific LG domains: LG3 induces osteogenic differentiation, but not mineralization.Exp Cell Res. 2008 Feb 15;314(4):763-73. doi: 10.1016/j.yexcr.2007.12.007. Epub 2008 Jan 22. Exp Cell Res. 2008. PMID: 18206871 Free PMC article.
References
-
- Grosenbaugh DA, Amoss MS, Hood DM, Morgan SJ, Williams JD. Epidermal growth factor-mediated effects on equine vascular smooth muscle cells. Am J Physiol. 1988;255:C447–C451. - PubMed
-
- Klagsbrun M, Edelman ER. Biological and biochemical properties of fibroblast growth factors: implications for the pathogenesis of atherosclerosis. Arteriosclerosis. 1989;9:269–278. - PubMed
-
- Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–318. - PubMed
LinkOut - more resources
Full Text Sources

