Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina
- PMID: 15683564
- PMCID: PMC1847781
- DOI: 10.1017/S0952523804215127
Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina
Abstract
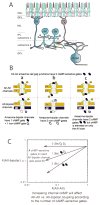
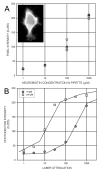
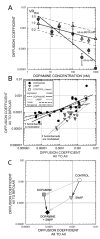
Gap junctions are commonplace in retina, often between cells of the same morphological type, but sometimes linking different cell types. The strength of coupling between cells derives from the properties of the connexins, but also is regulated by the intracellular environment of each cell. We measured the relative coupling of two different gap junctions made by AII amacrine cells of the rabbit retina. Permeability to the tracer Neurobiotin was measured at different concentrations of the neuromodulators dopamine, nitric oxide, or cyclic adenosine monophosphate (cAMP) analogs. Diffusion coefficients were calculated separately for the gap junctions between pairs of AII amacrine cells and for those connecting AII amacrine cells with ON cone bipolar cells. Increased dopamine caused diffusion rates to decline more rapidly across the AII-AII gap junctions than across the AII-bipolar cell gap junctions. The rate of decline at these sites was well fit by a model proposing that dopamine modulates two independent gates in AII-AII channels, but only a single gate on the AII side of the AII-bipolar channel. However, a membrane-permeant cAMP agonist modulated both types of channel equally. Therefore, the major regulator of channel closure in this network is the local cAMP concentration within each cell, as regulated by dopamine, rather than different cAMP sensitivity of their respective gates. In contrast, nitric oxide preferentially reduced AII-bipolar cell permeabilities. Coupling from AII amacrine cells to the different bipolar cell subtypes was differentially affected by dopamine, indicating that light adaptation acting via dopamine release alters network coupling properties in multiple ways.
Figures


Similar articles
-
Differential properties of two gap junctional pathways made by AII amacrine cells.Nature. 1995 Oct 26;377(6551):734-7. doi: 10.1038/377734a0. Nature. 1995. PMID: 7477263
-
Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional.J Comp Neurol. 2001 Sep 3;437(4):408-22. doi: 10.1002/cne.1292. J Comp Neurol. 2001. PMID: 11503143
-
Gap junctions between AII amacrine cells and calbindin-positive bipolar cells in the rabbit retina.Vis Neurosci. 1999 Nov-Dec;16(6):1181-9. doi: 10.1017/s0952523899166173. Vis Neurosci. 1999. PMID: 10614597
-
Electrical synapses between AII amacrine cells in the retina: Function and modulation.Brain Res. 2012 Dec 3;1487:160-72. doi: 10.1016/j.brainres.2012.05.060. Epub 2012 Jul 7. Brain Res. 2012. PMID: 22776293 Review.
-
Multiple neuronal connexins in the mammalian retina.Cell Commun Adhes. 2003 Jul-Dec;10(4-6):425-30. doi: 10.1080/cac.10.4-6.425.430. Cell Commun Adhes. 2003. PMID: 14681052 Review.
Cited by
-
Motor patterns of the small intestine explained by phase-amplitude coupling of two pacemaker activities: the critical importance of propagation velocity.Am J Physiol Cell Physiol. 2015 Sep 15;309(6):C403-14. doi: 10.1152/ajpcell.00414.2014. Epub 2015 Jul 1. Am J Physiol Cell Physiol. 2015. PMID: 26135802 Free PMC article.
-
Scaling Our World View: How Monoamines Can Put Context Into Brain Circuitry.Front Cell Neurosci. 2018 Dec 20;12:506. doi: 10.3389/fncel.2018.00506. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30618646 Free PMC article.
-
Analytical methods for assessing retinal cell coupling using cut-loading.PLoS One. 2022 Jul 19;17(7):e0271744. doi: 10.1371/journal.pone.0271744. eCollection 2022. PLoS One. 2022. PMID: 35853039 Free PMC article.
-
Dopamine D1 receptor activation reduces local inner retinal inhibition to light-adapted levels.J Neurophysiol. 2019 Apr 1;121(4):1232-1243. doi: 10.1152/jn.00448.2018. Epub 2019 Feb 6. J Neurophysiol. 2019. PMID: 30726156 Free PMC article.
-
Components and properties of the G3 ganglion cell circuit in the rabbit retina.J Comp Neurol. 2009 Mar 1;513(1):69-82. doi: 10.1002/cne.21941. J Comp Neurol. 2009. PMID: 19107780 Free PMC article.
References
-
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Visual Neuroscience. 1997;14:565–576. - PubMed
-
- Chow CC, Kopell N. Dynamics of spiking neurons with electrical coupling. Neural Computation. 2000;12:1643–1678. - PubMed
-
- Cohen E, Sterling P. Demonstration of cell types among cone bipolar neutrons of cat retina. Philosophical Transactions of the Royal Society B (London) 1990;330:305–321. - PubMed
-
- Condorelli DF, Parenti R, Spinella F, Trovato Salinaro A, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. European Journal of Neuroscience. 1998;10:1202–1208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

