Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin
- PMID: 15684033
- PMCID: PMC2171731
- DOI: 10.1083/jcb.200407076
Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin
Abstract
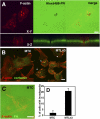
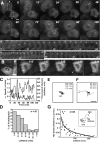
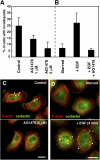
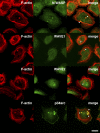
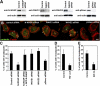
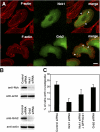
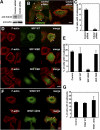
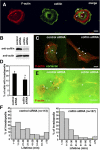
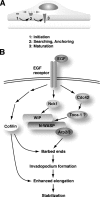
Invadopodia are actin-rich membrane protrusions with a matrix degradation activity formed by invasive cancer cells. We have studied the molecular mechanisms of invadopodium formation in metastatic carcinoma cells. Epidermal growth factor (EGF) receptor kinase inhibitors blocked invadopodium formation in the presence of serum, and EGF stimulation of serum-starved cells induced invadopodium formation. RNA interference and dominant-negative mutant expression analyses revealed that neural WASP (N-WASP), Arp2/3 complex, and their upstream regulators, Nck1, Cdc42, and WIP, are necessary for invadopodium formation. Time-lapse analysis revealed that invadopodia are formed de novo at the cell periphery and their lifetime varies from minutes to several hours. Invadopodia with short lifetimes are motile, whereas long-lived invadopodia tend to be stationary. Interestingly, suppression of cofilin expression by RNA interference inhibited the formation of long-lived invadopodia, resulting in formation of only short-lived invadopodia with less matrix degradation activity. These results indicate that EGF receptor signaling regulates invadopodium formation through the N-WASP-Arp2/3 pathway and cofilin is necessary for the stabilization and maturation of invadopodia.
Figures
References
-
- Bailly, M., L. Yan, G.M. Whitesides, J.S. Condeelis, and J.E. Segall. 1998. Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp. Cell Res. 241:285–299. - PubMed
-
- Banzai, Y., H. Miki, H. Yamaguchi, and T. Takenawa. 2000. Essential role of neural Wiskott-Aldrich syndrome protein in neurite extension in PC12 cells and rat hippocampal primary culture cells. J. Biol. Chem. 275:11987–11992. - PubMed
-
- Benesch, S., S. Lommel, A. Steffen, T.E. Stradal, N. Scaplehorn, M. Way, J. Wehland, and K. Rottner. 2002. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J. Biol. Chem. 277:37771–37776. - PubMed
-
- Buccione, R., J.D. Orth, and M.A. McNiven. 2004. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell Biol. 5:647–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

