A randomized, double-blind, placebo-controlled noninferiority trial of amoxicillin for clinically diagnosed acute otitis media in children 6 months to 5 years of age
- PMID: 15684116
- PMCID: PMC545757
- DOI: 10.1503/cmaj.1040771
A randomized, double-blind, placebo-controlled noninferiority trial of amoxicillin for clinically diagnosed acute otitis media in children 6 months to 5 years of age
Abstract
Objectives: Debate continues with respect to a "watch and wait" approach versus immediate antibiotic treatment for the initial treatment of acute otitis media. In this double-blind noninferiority trial, we compared clinical improvement rates at 14 days for children (6 months to 5 years of age) with acute otitis media who were randomly assigned to receive amoxicillin or placebo.
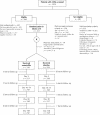
Methods: We enrolled healthy children who presented to clinics or the emergency department with a new episode of acute otitis media during the fall and winter months in Ottawa (from December 1999 to the end of March 2002). The children were randomly assigned to receive amoxicillin (60 mg/kg daily) or placebo for 10 days. Telephone follow-up was performed on each of days 1, 2 and 3 and once between day 10 and day 14. The primary outcome was clinical resolution of symptoms, defined as absence of receipt of an antimicrobial (other than the amoxicillin in the treatment group) at any time during the 14-day period. Secondary outcomes were the presence of pain and fever and the activity level in the first 3 days, recurrence rates, and the presence of middle ear effusion at 1 and 3 months.
Results: According to clinical scoring, 415 of the 512 children who could be evaluated had moderate disease. At 14 days 84.2% of the children receiving placebo and 92.8% of those receiving amoxicillin had clinical resolution of symptoms (absolute difference -8.6%, 95% confidence interval -14.4% to -3.0%). Children who received placebo had more pain and fever in the first 2 days. There were no statistical differences in adverse events between the 2 groups, nor were there any significant differences in recurrence rates or middle ear effusion at 1 and 3 months.
Interpretation: Our results did not support the hypothesis that placebo was noninferior to amoxicillin (i.e., that the 14-day cure rates among children with clinically diagnosed acute otitis media would not be substantially worse in the placebo group than the treatment group). Nevertheless, delaying treatment was associated with resolution of clinical signs and symptoms in most of the children.
Figures
Comment in
-
Antibiotic treatment for acute otitis media: time to think again.CMAJ. 2005 Mar 1;172(5):657-8. doi: 10.1503/cmaj.050078. CMAJ. 2005. PMID: 15738492 Free PMC article. No abstract available.
-
Treatment for otitis media.CMAJ. 2005 Aug 2;173(3):235; author reply 236. doi: 10.1503/cmaj.1050054. CMAJ. 2005. PMID: 16076800 Free PMC article. No abstract available.
-
Treatment for otitis media.CMAJ. 2005 Aug 2;173(3):235; author reply 236. doi: 10.1503/cmaj.1050050. CMAJ. 2005. PMID: 16076801 Free PMC article. No abstract available.
-
Treatment for otitis media.CMAJ. 2005 Aug 2;173(3):235; author reply 236. doi: 10.1503/cmaj.1050041. CMAJ. 2005. PMID: 16076802 Free PMC article. No abstract available.
-
Treatment for otitis media.CMAJ. 2005 Aug 2;173(3):236; author reply 236. doi: 10.1503/cmaj.1050051. CMAJ. 2005. PMID: 16076805 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
