Synergistic role of micronemal proteins in Toxoplasma gondii virulence
- PMID: 15684324
- PMCID: PMC2213027
- DOI: 10.1084/jem.20041672
Synergistic role of micronemal proteins in Toxoplasma gondii virulence
Abstract
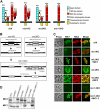
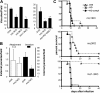
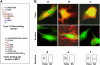
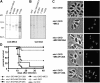
Apicomplexan parasites invade cells by a unique mechanism involving discharge of secretory vesicles called micronemes. Microneme proteins (MICs) include transmembrane and soluble proteins expressing different adhesive domains. Although the transmembrane protein TRAP and its homologues are thought to bridge cell surface receptors and the parasite submembranous motor, little is known about the function of other MICs. We have addressed the role of MIC1 and MIC3, two soluble adhesins of Toxoplasma gondii, in invasion and virulence. Single deletion of the MIC1 gene decreased invasion in fibroblasts, whereas MIC3 deletion had no effect either alone or in the mic1KO context. Individual disruption of MIC1 or MIC3 genes slightly reduced virulence in the mouse, whereas doubly depleted parasites were severely impaired in virulence and conferred protection against subsequent challenge. Single substitution of two critical amino acids in the chitin binding-like (CBL) domain of MIC3 abolished MIC3 binding to cells and generated the attenuated virulence phenotype. Our findings identify the CBL domain of MIC3 as a key player in toxoplasmosis and reveal the synergistic role of MICs in virulence, supporting the idea that parasites have evolved multiple ligand-receptor interactions to ensure invasion of different cells types during the course of infection.
Figures

References
-
- Menard, R. 2001. Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cell. Microbiol. 3:63–73. - PubMed
-
- Soldati, D., J.F. Dubremetz, and M. Lebrun. 2001. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int. J. Parasitol. 31:1293–1302. - PubMed
-
- Tomley, F.M., and D.S. Soldati. 2001. Mix and match modules: structure and function of microneme proteins in apicomplexan parasites. Trends Parasitol. 17:81–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

