Structural basis for the function and regulation of the receptor protein tyrosine phosphatase CD45
- PMID: 15684325
- PMCID: PMC2213029
- DOI: 10.1084/jem.20041890
Structural basis for the function and regulation of the receptor protein tyrosine phosphatase CD45
Erratum in
- J Exp Med. 2005 Mar 21;201(6):1019
Abstract
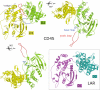
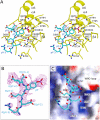
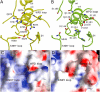
CD45 is the prototypic member of transmembrane receptor-like protein tyrosine phosphatases (RPTPs) and has essential roles in immune functions. The cytoplasmic region of CD45, like many other RPTPs, contains two homologous protein tyrosine phosphatase domains, active domain 1 (D1) and catalytically impaired domain 2 (D2). Here, we report crystal structure of the cytoplasmic D1D2 segment of human CD45 in native and phosphotyrosyl peptide-bound forms. The tertiary structures of D1 and D2 are very similar, but doubly phosphorylated CD3zeta immunoreceptor tyrosine-based activation motif peptide binds only the D1 active site. The D2 "active site" deviates from the other active sites significantly to the extent that excludes any possibility of catalytic activity. The relative orientation of D1 and D2 is very similar to that observed in leukocyte common antigen-related protein with both active sites in an open conformation and is restrained through an extensive network of hydrophobic interactions, hydrogen bonds, and salt bridges. This crystal structure is incompatible with the wedge model previously suggested for CD45 regulation.
Figures

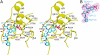
Similar articles
-
Intra- and intermolecular interactions between intracellular domains of receptor protein-tyrosine phosphatases.J Biol Chem. 2002 Dec 6;277(49):47263-9. doi: 10.1074/jbc.M205810200. Epub 2002 Oct 9. J Biol Chem. 2002. PMID: 12376545
-
Crystal structure of the tandem phosphatase domains of RPTP LAR.Cell. 1999 May 14;97(4):449-57. doi: 10.1016/s0092-8674(00)80755-2. Cell. 1999. PMID: 10338209
-
Subdomain X of the kinase domain of Lck binds CD45 and facilitates dephosphorylation.J Biol Chem. 2004 Jan 30;279(5):3455-62. doi: 10.1074/jbc.M309537200. Epub 2003 Nov 17. J Biol Chem. 2004. PMID: 14625311
-
Probing the phosphopeptide specificities of protein tyrosine phosphatases, SH2 and PTB domains with combinatorial library methods.Curr Protein Pept Sci. 2002 Aug;3(4):365-97. doi: 10.2174/1389203023380594. Curr Protein Pept Sci. 2002. PMID: 12370002 Review.
-
New insights into the transmembrane protein tyrosine phosphatase CD45.Int J Biochem Cell Biol. 2001 Nov;33(11):1041-6. doi: 10.1016/s1357-2725(01)00075-9. Int J Biochem Cell Biol. 2001. PMID: 11551820 Review.
Cited by
-
Protein tyrosine phosphatases--from housekeeping enzymes to master regulators of signal transduction.FEBS J. 2013 Jan;280(2):346-78. doi: 10.1111/febs.12077. Epub 2013 Jan 17. FEBS J. 2013. PMID: 23176256 Free PMC article. Review.
-
Structure of the catalytic domain of protein tyrosine phosphatase sigma in the sulfenic acid form.Mol Cells. 2013 Jul;36(1):55-61. doi: 10.1007/s10059-013-0033-x. Epub 2013 May 30. Mol Cells. 2013. PMID: 23820885 Free PMC article.
-
Protein tyrosine phosphatases in lymphocyte activation and autoimmunity.Nat Immunol. 2012 Apr 18;13(5):439-47. doi: 10.1038/ni.2246. Nat Immunol. 2012. PMID: 22513334 Review.
-
Enforced Dimerization of CD45 by the Adenovirus E3/49K Protein Inhibits T Cell Receptor Signaling.J Virol. 2023 May 31;97(5):e0189822. doi: 10.1128/jvi.01898-22. Epub 2023 May 1. J Virol. 2023. PMID: 37125921 Free PMC article.
-
Predicting protein interactions of the kinase Lck critical to T cell modulation.Structure. 2024 Nov 7;32(11):2168-2179.e2. doi: 10.1016/j.str.2024.09.010. Epub 2024 Oct 4. Structure. 2024. PMID: 39368461
References
-
- Alonso, A., J. Sasin, N. Bottini, I. Friedberg, I. Friedberg, A. Osterman, A. Godzik, T. Hunter, J. Dixon, and T. Mustelin. 2004. Protein tyrosine phosphatases in the human genome. Cell. 117:699–711. - PubMed
-
- Tonks, N.K., H. Charbonneau, C.D. Diltz, E.H. Fischer, and K.A. Walsh. 1988. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 27:8695–8701. - PubMed
-
- Trowbridge, I.S., and M.L. Thomas. 1994. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu. Rev. Immunol. 12:85–116. - PubMed
-
- Hermiston, M.L., Z. Xu, and A. Weiss. 2003. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 21:107–137. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

