A potential role for extracellular nitric oxide generation in cGMP-independent inhibition of human platelet aggregation: biochemical and pharmacological considerations
- PMID: 15685209
- PMCID: PMC1576067
- DOI: 10.1038/sj.bjp.0706110
A potential role for extracellular nitric oxide generation in cGMP-independent inhibition of human platelet aggregation: biochemical and pharmacological considerations
Abstract
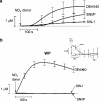
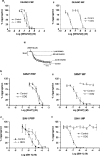
1. Nitric oxide (NO) is a potent inhibitor of platelet activation, that inhibits the agonist-induced increase in cytosolic Ca2+ concentration through both cGMP-dependent and independent pathways. However, the NO-related (NOx) species responsible for cGMP-independent signalling in platelets is unclear. We tested the hypothesis that extracellular NO, but not NO+ or peroxynitrite, generated in the extracellular compartment is responsible for cGMP-independent inhibition of platelet activation via inhibition of Ca2+ signalling. 2. Concentration-response curves for diethylamine diazeniumdiolate (DEA/NO; a spontaneous NO generator), S-nitroso-N-valerylpenicillamine (SNVP; an S-nitrosothiol) and 3-morpholinosydnonomine (SIN-1; a peroxynitrite generator) were generated in platelet-rich plasma (PRP) and washed platelets (WP) in the presence and absence of a supramaximal concentration of the soluble guanylate cyclase inhibitor, ODQ (20 microM). All three NOx donors displayed cGMP-independent inhibition of platelet aggregation in PRP, but only DEA/NO exhibited cGMP-independent inhibition of aggregation in WP. 3. Analysis of NO generation using an isolated NO-electrode revealed that cGMP-independent effects coincided with the generation of substantial levels of extracellular NO (>40 nM) from the NOx donors. 4. Reconstitution of WP with plasma factors indicated that the copper-containing plasma protein, caeruloplasmin (CP), catalysed the release of NO from SNVP, while Cu/Zn superoxide dismutase (SOD) unmasked NO generated from SIN-1. The increased generation of extracellular NO correlated with a switch to cGMP-independent effects with both NOx donors. 5. Analysis of Fura-2 loaded WP revealed that only DEA/NO inhibited Ca2+ signalling in platelets via a cGMP-independent mechanism. However, preincubation of SNVP and SIN-1 with CP and SOD, respectively, induced cGMP-independent inhibition of intraplatelet Ca2+ trafficking by the NOx donors. 6. Taken together, our data suggest that extracellular NO (>40 nM) is required for cGMP-independent inhibition of platelet activation. Plasma constituents may play an important pharmacological role in activating cGMP-independent signalling by S-nitrosothiols or peroxynitrite generators.
Figures




Similar articles
-
Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms.Biochem Biophys Res Commun. 2000 Dec 20;279(2):412-9. doi: 10.1006/bbrc.2000.3976. Biochem Biophys Res Commun. 2000. PMID: 11118301
-
Evidence for a cyclic GMP-independent mechanism in the anti-platelet action of S-nitrosoglutathione.Br J Pharmacol. 1998 May;124(1):141-8. doi: 10.1038/sj.bjp.0701821. Br J Pharmacol. 1998. PMID: 9630353 Free PMC article.
-
In vitro inhibition of human and rat platelets by NO donors, nitrosoglutathione, sodium nitroprusside and SIN-1, through activation of cGMP-independent pathways.Pharmacol Res. 2011 Sep;64(3):289-97. doi: 10.1016/j.phrs.2011.03.014. Epub 2011 Apr 23. Pharmacol Res. 2011. PMID: 21539916
-
Evidence for, and importance of, cGMP-independent mechanisms with NO and NO donors on blood vessels and platelets.Curr Vasc Pharmacol. 2005 Jan;3(1):41-53. doi: 10.2174/1570161052773933. Curr Vasc Pharmacol. 2005. PMID: 15638781 Review.
-
Antiplatelet and antithrombotic effects of organic nitrates.Am J Cardiol. 1992 Sep 24;70(8):18B-22B. doi: 10.1016/0002-9149(92)90590-u. Am J Cardiol. 1992. PMID: 1529922 Review.
Cited by
-
S-nitrosylation in cardiovascular signaling.Circ Res. 2010 Mar 5;106(4):633-46. doi: 10.1161/CIRCRESAHA.109.207381. Circ Res. 2010. PMID: 20203313 Free PMC article. Review.
-
Routes for formation of S-nitrosothiols in blood.Cell Biochem Biophys. 2013 Nov;67(2):385-98. doi: 10.1007/s12013-011-9321-2. Cell Biochem Biophys. 2013. PMID: 22161622 Free PMC article. Review.
-
Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase.Proc Natl Acad Sci U S A. 2007 May 1;104(18):7699-704. doi: 10.1073/pnas.0609778104. Epub 2007 Apr 23. Proc Natl Acad Sci U S A. 2007. PMID: 17452643 Free PMC article.
-
Extra-platelet low-molecular-mass thiols mediate the inhibitory action of S-nitrosoalbumin on human platelet aggregation via S-transnitrosylation of the platelet surface.Amino Acids. 2021 Apr;53(4):563-573. doi: 10.1007/s00726-021-02950-8. Epub 2021 Feb 14. Amino Acids. 2021. PMID: 33586042 Free PMC article.
-
S-Nitrosoglutathione improves haemodynamics in early-onset pre-eclampsia.Br J Clin Pharmacol. 2014 Sep;78(3):660-9. doi: 10.1111/bcp.12379. Br J Clin Pharmacol. 2014. PMID: 24627995 Free PMC article. Clinical Trial.
References
-
- AHERN G.P., KLYACHKO V.A., JACKSON M.B. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–517. - PubMed
-
- BLOCKMANS D., DECKMYN H., VERMYLEN J. Platelet activation. Blood Rev. 1995;9:143–156. - PubMed
-
- BROWN A.S., MORO M.A., MASSE J.M., CRAMER E.M., RADOMSKI M., DARLEY-USMAR V. Nitric oxide-dependent and independent effects on human platelets treated with peroxynitrite. Cardiovasc. Res. 1998;40:380–388. - PubMed
-
- BUSSE R., LUCKHOFF A., BASSENGE E. Endothelium-derived relaxant factor inhibits platelet activation. Naunyn Schmiedebergs Arch. Pharmacol. 1987;336:566–571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

