An electrophysiological study of excitatory purinergic neuromuscular transmission in longitudinal smooth muscle of chicken anterior mesenteric artery
- PMID: 15685211
- PMCID: PMC1576065
- DOI: 10.1038/sj.bjp.0706076
An electrophysiological study of excitatory purinergic neuromuscular transmission in longitudinal smooth muscle of chicken anterior mesenteric artery
Abstract
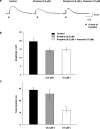
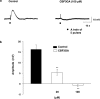
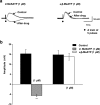
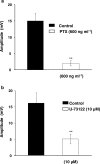
1. The object of the present study was to clarify the neurotransmitters controlling membrane responses to electrical field stimulation (EFS) in the longitudinal smooth muscle cells of the chicken anterior mesenteric artery. 2. EFS (5 pulses at 20 Hz) evoked a depolarization of amplitude 19.7+/-2.1 mV, total duration 29.6+/-3.1 s and latency 413.0+/-67.8 ms. This depolarization was tetrodotoxin (TTX)-sensitive and its amplitude was partially decreased by atropine (0.5 microM); however, its duration was shortened by further addition of prazosin (10 microM). 3. Atropine/prazosin-resistant component was blocked by the nonspecific purinergic antagonist, suramin, in a dose-dependent manner, indicating that this component is mediated by the neurotransmitter adenosine 5'-triphosphate (ATP). 4. Neither desensitization nor blocking of P2X receptor with its putative receptor agonist alpha,beta-methylene ATP (alpha,beta-MeATP, 1 microM) and its antagonist pyridoxalphosphate-6-azophenyl-2',4'-disulfonic (PPADS, up to 50 microM), had significant effect on the purinergic depolarization. In contrast, either desensitization or blocking of P2Y receptor with its putative agonist 2-methylthioATP (2-MeSATP, 1 microM) and its antagonist Cibacron blue F3GA (CBF3GA, 10 microM) abolished the purinergic depolarization, indicating that this response is mediated through P2Y but not P2X receptor. 5. The purinergic depolarization was inhibited by pertussis toxin (PTX, 600 ng ml(-1)). Furthermore, it was significantly inhibited by a phospholipase C (PLC) inhibitor, U-73122 (10 microM), indicating that the receptors involved in mediating the purinergic depolarization are linked to a PTX-sensitive G-protein, which is involved in a PLC-mediated signaling pathway. 6. Data of the present study suggest that the EFS-induced excitatory membrane response occurring in the longitudinal smooth muscle of the chicken anterior mesenteric artery is mainly purinergic in nature and is mediated via P2Y purinoceptors.
Figures
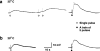


Similar articles
-
Neurally released ATP mediates endothelium-dependent hyperpolarization in the circular smooth muscle cells of chicken anterior mesenteric artery.Br J Pharmacol. 2005 Dec;146(7):983-9. doi: 10.1038/sj.bjp.0706413. Br J Pharmacol. 2005. PMID: 16231006 Free PMC article.
-
ATP released from perivascular nerves hyperpolarizes smooth muscle cells by releasing an endothelium-derived factor in hamster mesenteric arteries.J Physiol. 1999 Nov 15;521 Pt 1(Pt 1):191-9. doi: 10.1111/j.1469-7793.1999.00191.x. J Physiol. 1999. PMID: 10562344 Free PMC article.
-
Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels.Br J Pharmacol. 2000 Dec;131(8):1659-66. doi: 10.1038/sj.bjp.0703744. Br J Pharmacol. 2000. PMID: 11139444 Free PMC article.
-
Design and pharmacology of selective P2-purinoceptor antagonists.J Auton Pharmacol. 1996 Dec;16(6):341-4. doi: 10.1111/j.1474-8673.1996.tb00049.x. J Auton Pharmacol. 1996. PMID: 9131412 Review.
-
ATP as a cotransmitter in perivascular sympathetic nerves.J Auton Pharmacol. 1996 Dec;16(6):337-40. doi: 10.1111/j.1474-8673.1996.tb00048.x. J Auton Pharmacol. 1996. PMID: 9131411 Review.
Cited by
-
P2X purinoceptors mediate an endothelium-dependent hyperpolarization in longitudinal smooth muscle of anterior mesenteric artery in young chickens.Br J Pharmacol. 2009 Oct;158(3):888-95. doi: 10.1111/j.1476-5381.2009.00356.x. Epub 2009 Aug 19. Br J Pharmacol. 2009. PMID: 19694725 Free PMC article.
-
Development of longitudinal smooth muscle in the posterior mesenteric artery and purinergic regulation of its contractile responses in chickens.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Oct;199(10):857-65. doi: 10.1007/s00359-013-0848-0. Epub 2013 Aug 21. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 23963637
References
-
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol. Ther. 1994;64:445–475. - PubMed
-
- AKBAR G.K.M., DASARI V.R., WEBB T.E., AYYANATHAN K., PILLARISETTI K., SANDHU A.K., ATHWAL R.S., DANIEL J.L., ASHBY B., BARNARD E.A., KUNAPULI S.P. Molecular cloning of a novel P2 purinoceptor from human erythroleukemia cells. J. Biol. Chem. 1996;271:18363–18367. - PubMed
-
- BALL R.A., SAUTTER J.H., KATTER M.S. Morphological characteristics of the anterior mesenteric artery of fowl. Anat. Rec. 1963;146:251–255. - PubMed
-
- BAO J.X., ERIKSSON I.E., STJRANE I. On pre- and/or postjunctional roles of ATP and unknown ‘Substance X' as sympathetic co-transmitters in rat tail artery. Acta Physiol. Scand. 1989;135:65–66. - PubMed
-
- BAUER V., HOLZER P., ITO Y. Role of extra- and intracellular calcium in the contractile action of agonists in the guinea-pig ileum. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:58–64. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

