Validation of pharmacodynamic assays to evaluate the clinical efficacy of an antisense compound (AEG 35156) targeted to the X-linked inhibitor of apoptosis protein XIAP
- PMID: 15685240
- PMCID: PMC2362094
- DOI: 10.1038/sj.bjc.6602363
Validation of pharmacodynamic assays to evaluate the clinical efficacy of an antisense compound (AEG 35156) targeted to the X-linked inhibitor of apoptosis protein XIAP
Abstract
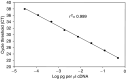
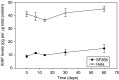
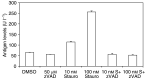
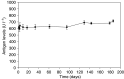
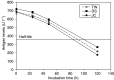
The inhibitor of apoptosis protein, XIAP, is frequently overexpressed in chemoresistant human tumours. An antisense oligonucleotide (AEG 35156/GEM 640) that targets XIAP has recently entered phase I trials in the UK. Method validation data are presented on three pharmacodynamic assays that will be utilised during this trial. Quantitative RT-PCR was based on a Taqman assay and was confirmed to be specific for XIAP. Assay linearity extended over four orders of magnitude. MDA-MB-231/U6-E1 cells and clone X-G4 stably expressing an RNAi vector against XIAP were chosen as high and low XIAP expression quality controls (QCs). Within-day and between-day coefficients of variation (CVs) in precision for cycle threshold (CT) and delta CT values (employing GAPDH and beta 2 microglobulin as housekeepers) were always less than 10%. A Western blotting technique was validated using a GST-XIAP fusion protein as a standard and HeLa cells and SF268 (human glioblastoma) cells as high and low XIAP expression QCs. Specificity of the final choice of antibody for XIAP was evaluated by analysing a panel of cell lines including clone X-G4. The assay was linear over a 29-fold range of protein concentration and between-day precision was 29% for the low QC and 23% for the high QC when normalised to GAPDH. XIAP protein was also shown to be stable at -80 degrees C for at least 60 days. M30-Apoptosense plasma Elisa detects a caspase-cleaved fragment of cytokeratin 18 (CK18), believed to be a surrogate marker for tumour cell apoptosis. Generation of an independent QC was achieved through the treatment of X-G4 cells with staurosporine and collection of media. Measurements on assay precision and kit-to-kit QC were always less than 10%. The M30 antigen (CK18-Asp396) was stable for 3 months at -80 degrees C, while at 37 degrees C it had a half-life of 80-100 h in healthy volunteer plasma. Results from the phase I trial are eagerly awaited.
Figures



References
-
- Bilim V, Kasahara T, Hara N, Takahashi K, Tomita Y (2003) Role of XIAP in the malignant phenotype of transitional cell cancer (TCC) and therapeutic activity of XIAP antisense oligonucleotides against multidrug-resistant TCC in vitro. Int J Cancer 103: 29–37 - PubMed
-
- Biven K, Erdal H, Hagg M, Ueno T, Zhou R, Lynch M, Rowley B, Wood J, Zhang C, Toi M, Shoshan MC, Linder S (2003) A novel assay for discovery and characterization of pro-apoptotic drugs and for monitoring apoptosis in patient sera. Apoptosis 8: 263–268 - PubMed
-
- Carr NJ (2000) M30 expression demonstrates apoptotic cells, correlates with in situ end-labeling, and is associated with Ki-67 expression in large intestinal neoplasms. Arch Pathol Lab Med 124: 1768–1772 - PubMed
-
- Dumur CI, Dechsukhum C, Wilkinson DS, Garrett CT, Ware JL, Ferreira-Gonzalez A (2002) Analytical validation of a real-time reverse transcription–polymerase chain reaction quantitation of different transcripts of the Wilms' tumor suppressor gene (WT1). Anal Biochem 309: 127–136 - PubMed
-
- Ferreira CG, van der Valk P, Span SW, Ludwig I, Smit EF, Kruyt FA, Pinedo HM, van Tinteren H, Giaccone G (2001) Expression of X-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell lung cancer patients. Clin Cancer Res 7: 2468–2474 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

