Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects
- PMID: 15685244
- PMCID: PMC2362084
- DOI: 10.1038/sj.bjc.6602301
Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects
Abstract
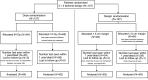
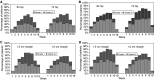
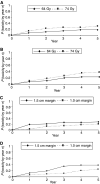
Radical radiotherapy is a standard form of management of localised prostate cancer. Conformal treatment planning spares adjacent normal tissues reducing treatment-related side effects and may permit safe dose escalation. We have tested the effects on tumour control and side effects of escalating radiotherapy dose and investigated the appropriate target volume margin. After an initial 3-6 month period of androgen suppression, 126 men were randomised and treated with radiotherapy using a 2 by 2 factorial trial design. The initial radiotherapy tumour target volume included the prostate and base of seminal vesicles (SV) or complete SV depending on SV involvement risk. Treatments were randomised to deliver a dose of 64 Gy with either a 1.0 or 1.5 cm margin around the tumour volume (1.0 and 1.5 cm margin groups) and also to treat either with or without a 10 Gy boost to the prostate alone with no additional margin (64 and 74 Gy groups). Tumour control was monitored by prostate-specific antigen (PSA) testing and clinical examination with additional tests as appropriate. Acute and late side effects of treatment were measured using the Radiation Treatment and Oncology Groups (RTOG) and LENT SOM systems. The results showed that freedom from PSA failure was higher in the 74 Gy group compared to the 64 Gy group, but this did not reach conventional levels of statistical significance with 5-year actuarial control rates of 71% (95% CI 58-81%) in the 74 Gy group vs 59% (95% CI 45-70%) in the 64 Gy group. There were 23 failures in the 74 Gy group and 33 in the 64 Gy group (Hazard ratio 0.64, 95% CI 0.38-1.10, P=0.10). No difference in disease control was seen between the 1.0 and 1.5 cm margin groups (5-year actuarial control rates 67%, 95% CI 53-77% vs 63%, 95% CI 50-74%) with 28 events in each group (Hazard ratio 0.97, 95% CI 0.50-1.86, P=0.94). Acute side effects were generally mild and 18 weeks after treatment, only four and five of the 126 men had persistent > or =Grade 1 bowel or bladder side effects, respectively. Statistically significant increases in acute bladder side effects were seen after treatment in the men receiving 74 Gy (P=0.006), and increases in both acute bowel side effects during treatment (P=0.05) and acute bladder sequelae (P=0.002) were recorded for men in the 1.5 cm margin group. While statistically significant, these differences were of short duration and of doubtful clinical importance. Late bowel side effects (RTOG> or =2) were seen more commonly in the 74 Gy and 1.5 cm margin groups (P=0.02 and P=0.05, respectively) in the first 2 years after randomisation. Similar results were found using the LENT SOM assessments. No significant differences in late bladder side effects were seen between the randomised groups using the RTOG scoring system. Using the LENT SOM instrument, a higher proportion of men treated in the 74 Gy group had Grade > or =3 urinary frequency at 6 and 12 months. Compared to baseline scores, bladder symptoms improved after 6 months or more follow-up in all groups. Sexual function deteriorated after treatment with the number of men reporting some sexual dysfunction (Grade> or =1) increasing from 38% at baseline to 66% at 6 months and 1 year and 81% by year 5. However, no consistent differences were seen between the randomised groups. In conclusion, dose escalation from 64 to 74 Gy using conformal radiotherapy may improve long-term PSA control, but a treatment margin of 1.5 cm is unnecessary and is associated with increased acute bowel and bladder reactions and more late rectal side effects. Data from this randomised pilot study informed the Data Monitoring Committee of the Medical Research Council RT 01 Trial and the two studies will be combined in subsequent meta-analysis.
Figures

References
-
- Amer AM, Mackay RI, Roberts SA, Hendry JH, Williams PC (2001) The required number of treatment imaging days for an effective off-line correction of systematic errors in conformal radiotherapy of prostate cancer – a radiobiological analysis. Radiother Oncol 61: 143–150 - PubMed
-
- Anacak Y, Yalman D, Ozsaran Z, Haydaroglu A (2001) Late radiation effects to the rectum and bladder in gynecologic cancer patients: the comparison of LENT/SOMA and RTOG/EORTC late-effects scoring systems. Int J Radiat Oncol Biol Phys 50: 1107–1112 - PubMed
-
- Bey P, Carrie C, Beckendorf V, Ginestet C, Aletti P, Madelis G, Luporsi E, Pommier P, Cowen D, Gonzague-Casabianca L, Simonian-Sauve M, Maingon P, Naudy S, Lagrange J, Marcie S (2000) Dose escalation with 3D-CRT in prostate cancer: French study of dose escalation with conformal 3D radiotherapy in prostate cancer-preliminary results. Int J Radiat Oncol Biol Phys 48: 513–517 - PubMed
-
- Boersma LJ, van den Brink M, Bruce AM, Shouman T, Gras L, te Velde A, Lebesque JV (1998) Estimation of the incidence of late bladder and rectum complications after high-dose (70–78 Gy) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys 41: 83–92, issn: 0360–3016 - PubMed
-
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Mattelaer J, Lopez Torecilla J, Pfeffer JR, Lino Cutajar C, Zurlo A, Pierart M (2002) Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360: 103–106 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

