The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves
- PMID: 15686598
- PMCID: PMC549193
- DOI: 10.1186/1471-2202-6-6
The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves
Abstract
Background: The progressive nature of Wallerian degeneration has long been controversial. Conflicting reports that distal stumps of injured axons degenerate anterogradely, retrogradely, or simultaneously are based on statistical observations at discontinuous locations within the nerve, without observing any single axon at two distant points. As axon degeneration is asynchronous, there are clear advantages to longitudinal studies of individual degenerating axons. We recently validated the study of Wallerian degeneration using yellow fluorescent protein (YFP) in a small, representative population of axons, which greatly improves longitudinal imaging. Here, we apply this method to study the progressive nature of Wallerian degeneration in both wild-type and slow Wallerian degeneration (WldS) mutant mice.
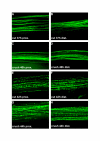
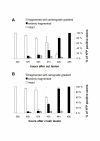
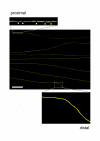
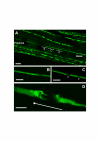
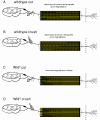
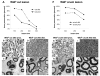
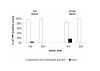
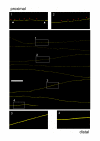
Results: In wild-type nerves, we directly observed partially fragmented axons (average 5.3%) among a majority of fully intact or degenerated axons 37-42 h after transection and 40-44 h after crush injury. Axons exist in this state only transiently, probably for less than one hour. Surprisingly, axons degenerated anterogradely after transection but retrogradely after a crush, but in both cases a sharp boundary separated intact and fragmented regions of individual axons, indicating that Wallerian degeneration progresses as a wave sequentially affecting adjacent regions of the axon. In contrast, most or all WldS axons were partially fragmented 15-25 days after nerve lesion, WldS axons degenerated anterogradely independent of lesion type, and signs of degeneration increased gradually along the nerve instead of abruptly. Furthermore, the first signs of degeneration were short constrictions, not complete breaks.
Conclusions: We conclude that Wallerian degeneration progresses rapidly along individual wild-type axons after a heterogeneous latent phase. The speed of progression and its ability to travel in either direction challenges earlier models in which clearance of trophic or regulatory factors by axonal transport triggers degeneration. WldS axons, once they finally degenerate, do so by a fundamentally different mechanism, indicated by differences in the rate, direction and abruptness of progression, and by different early morphological signs of degeneration. These observations suggest that WldS axons undergo a slow anterograde decay as axonal components are gradually depleted, and do not simply follow the degeneration pathway of wild-type axons at a slower rate.
Figures





References
-
- Waller A. Experiments on the section of glossopharyngeal and hypoglossal nerves of the frog and observations of the alternatives produced thereby in the structure of their primitive fibers. Philos Trans R Soc Lond Biol. 1850;140:423.
-
- Glass JD, Brushart TM, George EB, Griffin JW. Prolonged survival of transected nerve fibres in C57BL/Ola mice is an intrinsic characteristic of the axon. J Neurocytol. 1993;22:311–321. - PubMed
-
- Buckmaster EA, Perry VH, Brown MC. The rate of Wallerian degeneration in cultured neurons from wild type and C57BL/WldS mice depends on time in culture and may be extended in the presence of elevated K+ levels. Eur J Neurosci. 1995;7:1596–1602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

