The BvgAS signal transduction system regulates biofilm development in Bordetella
- PMID: 15687212
- PMCID: PMC545624
- DOI: 10.1128/JB.187.4.1474-1484.2005
The BvgAS signal transduction system regulates biofilm development in Bordetella
Abstract
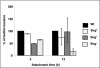
The majority of Bordetella sp. virulence determinants are regulated by the BvgAS signal transduction system. BvgAS mediates the control of multiple phenotypic phases and a spectrum of gene expression profiles specific to each phase in response to incremental changes in the concentrations of environmental signals. Studies highlighting the critical role of this signaling circuitry in the Bordetella infectious cycle have focused on planktonically growing bacterial cells. It is becoming increasingly clear that the major mode of bacterial existence in the environment and within the body is a surface-attached state known as a biofilm. Biofilms are defined as consortia of sessile microorganisms that are embedded in a matrix. During routine growth of Bordetella under agitating conditions, we noticed the formation of a bacterial ring at the air-liquid interface of the culture tubes. We show here that this surface adherence property reflects the ability of these organisms to form biofilms. Our data demonstrate that the BvgAS locus regulates biofilm development in Bordetella. The results reported in this study suggest that the Bvg-mediated control in biofilm development is exerted at later time points after the initial attachment of bacteria to the different surfaces. Additionally, we show that these biofilms are highly tolerant of a number of antimicrobials, including the ones that are currently recommended for treatment of veterinary and human infections caused by Bordetella spp. Finally, we discuss the significance of the biofilm lifestyle mode as a potential contributor to persistent infections.
Figures



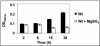

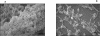
Similar articles
-
The Bvg virulence control system regulates biofilm formation in Bordetella bronchiseptica.J Bacteriol. 2004 Sep;186(17):5692-8. doi: 10.1128/JB.186.17.5692-5698.2004. J Bacteriol. 2004. PMID: 15317773 Free PMC article.
-
The bvg-repressed gene brtA, encoding biofilm-associated surface adhesin, is expressed during host infection by Bordetella bronchiseptica.Microbiol Immunol. 2016 Feb;60(2):93-105. doi: 10.1111/1348-0421.12356. Microbiol Immunol. 2016. PMID: 26756546
-
Lonidamine, a Novel Modulator for the BvgAS System of Bordetella Species.Microbiol Immunol. 2025 Mar;69(3):133-147. doi: 10.1111/1348-0421.13193. Epub 2024 Dec 15. Microbiol Immunol. 2025. PMID: 39674913 Free PMC article.
-
Environmental sensing mechanisms in Bordetella.Adv Microb Physiol. 2001;44:141-81. doi: 10.1016/s0065-2911(01)44013-6. Adv Microb Physiol. 2001. PMID: 11407112 Review.
-
Bordetella biofilms: a lifestyle leading to persistent infections.Pathog Dis. 2016 Feb;74(1):ftv108. doi: 10.1093/femspd/ftv108. Epub 2015 Nov 19. Pathog Dis. 2016. PMID: 26586694 Free PMC article. Review.
Cited by
-
Role of a putative polysaccharide locus in Bordetella biofilm development.J Bacteriol. 2007 Feb;189(3):750-60. doi: 10.1128/JB.00953-06. Epub 2006 Nov 17. J Bacteriol. 2007. PMID: 17114249 Free PMC article.
-
Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice.PLoS One. 2011 Feb 11;6(2):e16861. doi: 10.1371/journal.pone.0016861. PLoS One. 2011. PMID: 21347299 Free PMC article.
-
Bordetella adenylate cyclase toxin interacts with filamentous haemagglutinin to inhibit biofilm formation in vitro.Mol Microbiol. 2017 Jan;103(2):214-228. doi: 10.1111/mmi.13551. Epub 2016 Nov 3. Mol Microbiol. 2017. PMID: 27731909 Free PMC article.
-
The Bordetella Bps polysaccharide is critical for biofilm development in the mouse respiratory tract.J Bacteriol. 2007 Nov;189(22):8270-6. doi: 10.1128/JB.00785-07. Epub 2007 Jun 22. J Bacteriol. 2007. PMID: 17586629 Free PMC article.
-
Cross-species protection mediated by a Bordetella bronchiseptica strain lacking antigenic homologs present in acellular pertussis vaccines.Infect Immun. 2010 May;78(5):2008-16. doi: 10.1128/IAI.01142-09. Epub 2010 Feb 22. Infect Immun. 2010. PMID: 20176797 Free PMC article.
References
-
- Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella host interaction. Cell 80:611-620. - PubMed
-
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
-
- Bartkus, J. M., B. A. Juni, K. Ehresmann, C. A. Miller, G. N. Sanden, P. K. Cassiday, M. Saubolle, B. Lee, J. Long, A. R. Harrison, Jr., and J. M. Besser. 2003. Identification of a mutation associated with erythromycin resistance in Bordetella pertussis: implications for surveillance of antimicrobial resistance J. Clin. Microbiol. 41:1167-1172. - PMC - PubMed
-
- Birkebaek, N. H., M. Kristiansen, T. Seefeldt, J. Degn, A. Moller, I. Heron, P. L. Andersen, J. K. Moller, and L. Ostergard. 1999. Bordetella pertussis and chronic cough in adults. Clin. Infect. Dis. 29:1239-1242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

