Small-molecule XIAP inhibitors derepress downstream effector caspases and induce apoptosis of acute myeloid leukemia cells
- PMID: 15687241
- PMCID: PMC1895077
- DOI: 10.1182/blood-2004-08-3168
Small-molecule XIAP inhibitors derepress downstream effector caspases and induce apoptosis of acute myeloid leukemia cells
Abstract
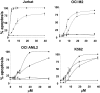
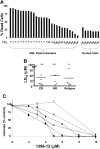
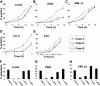
We tested the effects of small-molecule XIAP antagonists based on a polyphenylurea pharmacophore on cultured acute myelogenous leukemia (AML) cell lines and primary patient samples. X-linked inhibitor of apoptosis protein (XIAP) antagonist N-[(5R)-6-[(anilinocarbonyl)amino]-5-((anilinocarbonyl){[(2R)-1-(4-cyclohexylbutyl)pyrrolidin-2-yl]methyl}amino)hexyl]-N-methyl-N'-phenylurea (1396-12), but not a structurally related control compound, induced apoptosis of primary leukemia samples with a lethal dose (LD50) of less than 10 microM in 16 of 27 (60%) samples. In contrast, XIAP antagonist 1396-12 was not lethal to the normal hematopoietic cells in short-term cytotoxicity assays. Response of primary AML specimens to XIAP inhibitor correlated with XIAP protein levels, with higher levels of XIAP associated with sensitivity. The XIAP antagonist 1396-12 induced activation of downstream caspases 3 and 7 prior to the activation of upstream caspase 8 and caspase 9. Apoptosis induction was also independent of B-cell lymphoma protein-2 (Bcl-2) or caspase 8, indicative of a downstream effect on apoptotic pathways. Thus, polyphenylurea-based XIAP antagonsists directly induce apoptosis of leukemia cells and AML patient samples at low micromolar concentrations through a mechanism of action distinct from conventional chemotherapeutic agents.
Figures



References
-
- Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1: 19-30. - PubMed
-
- Cryns V, Yuan Y. Proteases to die for. Genes Dev. 1999;12: 1551-1570. - PubMed
-
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281: 1312-1316. - PubMed
-
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell death proteases. Nature. 1997;388: 300-304. - PubMed
-
- Liu Z, Sun C, Olejniczak ET, et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408: 1004-1008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

