A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73
- PMID: 15687255
- PMCID: PMC546509
- DOI: 10.1101/gad.319905
A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73
Abstract
Protein degradation is an essential and highly regulated process. The proteasomal degradation of the tumor suppressors p53 and p73 is regulated by both polyubiquitination and by an ubiquitin-independent process. Here, we show that this ubiquitin-independent process is mediated by the 20S proteasomes and is regulated by NQO1. NQO1 physically interacts with p53 and p73 in an NADH-dependent manner and protects them from 20S proteasomal degradation. Remarkably, the vast majority of NQO1 in cells is found in physical association with the 20S proteasomes, suggesting that NQO1 functions as a gatekeeper of the 20S proteasomes. We further show that this pathway plays a role in p53 accumulation in response to ionizing radiation. Our findings provide the first evidence for in vivo degradation of p53 and p73 by the 20S proteasomes and its regulation by NQO1 and NADH level.
Figures
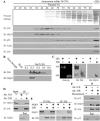

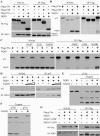


References
-
- Anwar A., Dehn, D., Siegel, D., Kepa, J.K., Tang, L.J., Pietenpol, J.A., and Ross, D. 2003. Interaction of human NAD(P)H:quinone oxidoreductase 1 (NQO1) with the tumor suppressor protein p53 in cells and cell-free systems. J. Biol. Chem. 278: 10368-10373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
