Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor
- PMID: 15687379
- PMCID: PMC548372
- DOI: 10.1093/nar/gni013
Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor
Abstract
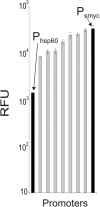
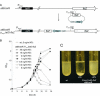
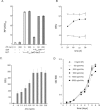
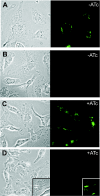
Gene expression systems that allow the regulation of bacterial genes during an infection are valuable molecular tools but are lacking for mycobacterial pathogens. We report the development of mycobacterial gene regulation systems that allow controlling gene expression in fast and slow-growing mycobacteria, including Mycobacterium tuberculosis, using anhydrotetracycline (ATc) as inducer. The systems are based on the Escherichia coli Tn10-derived tet regulatory system and consist of a strong tet operator (tetO)-containing mycobacterial promoter, expression cassettes for the repressor TetR and the chemical inducer ATc. These systems allow gene regulation over two orders of magnitude in Mycobacterium smegmatis and M.tuberculosis. TetR-controlled gene expression was inducer concentration-dependent and maximal with ATc concentrations at least 10- and 20-fold below the minimal inhibitory concentration for M.smegmatis and M.tuberculosis, respectively. Using the essential mycobacterial gene ftsZ, we showed that these expression systems can be used to construct conditional knockouts and to analyze the function of essential mycobacterial genes. Finally, we demonstrated that these systems allow gene regulation in M.tuberculosis within the macrophage phagosome.
Figures

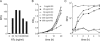
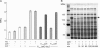

References
-
- Barnes P.F., Cave M.D. Molecular epidemiology of tuberculosis. N. Engl. J. Med. 2003;349:1149–1156. - PubMed
-
- Frieden T.R., Sterling T.R., Munsiff S.S., Watt C.J., Dye C. Tuberculosis. Lancet. 2003;362:887–899. - PubMed
-
- Kremer L., Besra G.S. Re-emergence of tuberculosis: strategies and treatment. Expert Opin. Investig. Drugs. 2002;11:153–157. - PubMed
-
- DeVito J.A., Mills J.A., Liu V.G., Agarwal A., Sizemore C.F., Yao Z., Stoughton D.M., Cappiello M.G., Barbosa M.D., Foster L.A., et al. An array of target-specific screening strains for antibacterial discovery. Nat. Biotechnol. 2002;20:478–483. - PubMed
-
- Mnaimneh S., Davierwala A.P., Haynes J., Moffat J., Peng W.T., Zhang W., Yang X., Pootoolal J., Chua G., Lopez A., et al. Exploration of essential gene functions via titratable promoter alleles. Cell. 2004;118:31–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

