Tetracycline-inducible gene regulation in mycobacteria
- PMID: 15687380
- PMCID: PMC548381
- DOI: 10.1093/nar/gni023
Tetracycline-inducible gene regulation in mycobacteria
Abstract
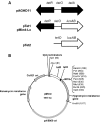
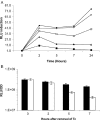
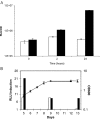
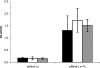
A system for the tetracycline-inducible regulation of gene expression in mycobacteria has been developed. We have sub-cloned the tetRO region from the Corynebacterium glutamicum TetZ locus into a mycobacterial shuttle plasmid, making expression of genes cloned downstream of tetRO responsive to tetracycline. Using the luxAB-encoded luciferase from Vibrio harveyi as a reporter (pMind-Lx), we observed a 40-fold increase in light output from Mycobacterium smegmatis cultures 2 h after adding 20 ng ml(-1) of tetracycline. Similarly, exposure to the drug resulted in up to 20-fold increase in relative light units from M.bovis BCG carrying the reporter construct, and a 10-fold increase for M.tuberculosis. Tetracycline induction was demonstrated in log and stationary phase cultures. To evaluate whether this system is amenable to use in vivo, J774 macrophages were infected with M.bovis BCG[pMind-Lx], treated with amikacin to kill extracellular bacteria, and then incubated with tetracycline. A 10-fold increase in light output was measured after 24 h, indicating that intracellular bacteria are accessible and responsive to exogenously added tetracycline. To test the use of the tetracycline-inducible system for conditional gene silencing, mycobacteria were transformed with a pMind construct with tetRO driving expression of antisense RNA for the ftsZ gene. Bacterial cells containing the antisense construct formed filaments after 24 h exposure to tetracycline. These results demonstrate the potential of this tetracycline-regulated system for the manipulation of mycobacterial gene expression inside and outside cells.
Figures

References
-
- Dye C., Scheele S., Dolin P., Pathania V., Raviglione R.C. Global burden of tuberculosis—estimated incidence, prevalence, and mortality by country. J. Am. Med. Assoc. 1999;282:677–686. - PubMed
-
- Wayne L.G., Sohaskey C.D. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 2001;55:139–163. - PubMed
-
- Capuano S.V., III, Croix D.A., Pawar S., Zinovik A., Myers A., Lin P.L., Bissel S., Fuhrman C., Klein E., Flynn J.L. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M.tuberculosis infection. Infect. Immun. 2003;71:5831–5844. - PMC - PubMed
-
- Flynn J.L., Capuano S.V., Croix D., Pawar S., Myers A., Zinovik A., Klein E. Non-human primates: a model for tuberculosis research. Tuberculosis (Edinb.) 2003;83:116–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

