Genetic and environmental determinants of bitter perception and sweet preferences
- PMID: 15687429
- PMCID: PMC1397914
- DOI: 10.1542/peds.2004-1582
Genetic and environmental determinants of bitter perception and sweet preferences
Abstract
Objective: Flavor is the primary dimension by which young children determine food acceptance. However, children are not merely miniature adults because sensory systems mature postnatally and their responses to certain tastes differ markedly from adults. Among these differences are heightened preferences for sweet-tasting and greater rejection of bitter-tasting foods. The present study tests the hypothesis that genetic variations in the newly discovered TAS2R38 taste gene as well as cultural differences are associated with differences in sensitivity to the bitter taste of propylthiouracil (PROP) and preferences for sucrose and sweet-tasting foods and beverages in children and adults.
Design: Genomic DNA was extracted from cheek cells of a racially and ethnically diverse sample of 143 children and their mothers. Alleles of the gene TAS2R38 were genotyped. Participants were grouped by the first variant site, denoted A49P, because the allele predicts a change from the amino acid alanine (A) to proline (P) at position 49. Henceforth, individuals who were homozygous for the bitter-insensitive allele are referred to as AA, those who were heterozygous for the bitter-insensitive allele are referred to as AP, and those who were homozygous for the bitter-sensitive allele are referred to as PP. Using identical procedures for children and mothers, PROP sensitivity and sucrose preferences were assessed by using forced-choice procedures that were embedded in the context of games that minimized the impact of language development and were sensitive to the cognitive limitations of pediatric populations. Participants were also asked about their preferences in cereals and beverages, and mothers completed a standardized questionnaire that measured various dimensions of their children's temperament.
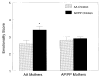
Results: Genetic variation of the A49P allele influenced bitter perception in children and adults. However, the phenotype-genotype relationship was modified by age such that 64% of heterozygous children, but only 43% of the heterozygous mothers, were sensitive to the lowest concentration (56 micromoles/liter) of PROP. Genotypes at the TAS2R38 locus were significantly related to preferences for sucrose and for sweet-tasting beverages and foods such as cereals in children. AP and PP children preferred significantly higher concentrations of sucrose solutions than did AA children. They were also significantly less likely to include milk or water as 1 of their 2 favorite beverages (18.6% vs 40%) and were more likely to include carbonated beverages as 1 of their most preferred beverages (46.4% vs 28.9%). PP children liked cereals and beverages with a significantly higher sugar content. There were also significant main effects of race/ethnicity on preferences and food habits. As a group, black children liked cereals with a significantly higher sugar content than did white children, and they were also significantly more likely to report that they added sugar to their cereals. Unlike children, there was no correspondence between TAS2R38 genotypes and sweet preference in adults. Here, the effects of race/ethnicity were the strongest determinants, thus suggesting that cultural forces and experience may override this genotype effect on sweet preferences. Differences in taste experiences also affected mother-child interaction, especially when the 2 resided in different sensory worlds. That is, children who had 1 or 2 bitter-sensitive alleles, but whose mothers had none, were perceived by their mothers as being more emotional than children who had no bitter-sensitive alleles.
Conclusion: Variations in a taste receptor gene accounted for a major portion of individual differences in PROP bitterness perception in both children and adults, as well as a portion of individual differences in preferences for sweet flavors in children but not in adults. These findings underscore the advantages of studying genotype effects on behavioral outcomes in children, especially as they relate to taste preferences because cultural forces may sometimes override the A49P genotypic effects in adults. New knowledge about the molecular basis of food likes and dislikes in children, a generation that will struggle with obesity and diabetes, may suggest strategies to overcome diet-induced diseases.
Conflict of interest statement
No conflict of interest declared.
Figures

References
-
- Mennella JA. Taste and smell. In: Swaiman KF, Ashwall S, eds. Pediatric Neurology: Principles and Practice 3rd ed. Philadelphia, PA: CV Mosby Company; 1999:104–113
-
- Desor JA, Maller O, Andrews K. Ingestive responses of human newborns to salty, sour, and bitter stimuli. J Comp Physiol Psychol. 1975;89:966–970. - PubMed
-
- Desor JA, Maller O, Turner RE. Preference for sweet in humans: infants, children and adults. In: Weiffenbach JM, ed. Taste and Development: The Genesis of Sweet Preference. Washington, DC: US Government Printing Office; 1977
-
- Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav. 1994;56:1217–1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

