Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines
- PMID: 15689172
- PMCID: PMC3180873
- DOI: 10.1021/jm0494422
Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines
Abstract
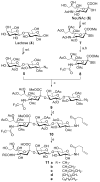
The problem of immunotolerance to GM3, an important tumor-associated trisaccharide antigen, seriously hinders its usage in cancer vaccine development. To solve this problem, the keyhole limpet hemocyanin (KLH) conjugates of a series of GM3 derivatives were synthesized and screened as therapeutic cancer vaccines. First, the beta-linked anomeric azides of differently N-acylated GM3 analogues were prepared by a highly convergent procedure. Next, a pentenoyl group was linked to the reducing end of the carbohydrate antigens following selective reduction of the azido group. The linker was thereafter ozonolyzed to give an aldehyde functionality permitting the conjugation of the antigens to KLH via reductive amination. Finally, the immunological properties of the resultant glycoconjugates were studied in C57BL/6 mice by assessing the titers of specific antibodies induced by the GM3 analogues. While KLH-GM3 elicited low levels of immune response, the KLH conjugates of N-propionyl, N-butanoyl, N-iso-butanoyl, and N-phenylacetyl GM3s induced robust immune reactions with antibodies of multiple isotypes, indicating significantly improved and T-cell dependent immune responses that lead to isotype switching, affinity maturation, and the induction of immunological "memory". It was suggested that GM3PhAc-KLH is a promising vaccine candidate for glycoengineered immunotherapy of cancer with GM3 as the primary target.
Figures





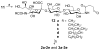
Similar articles
-
Preparation and immunological studies of protein conjugates of N -acylneuraminic acids.Glycoconj J. 2004;20(6):407-14. doi: 10.1023/B:GLYC.0000033997.01760.b9. Glycoconj J. 2004. PMID: 15238705 Free PMC article.
-
Vaccines prepared with sialyl-Tn and sialyl-Tn trimers using the 4-(4-maleimidomethyl)cyclohexane-1-carboxyl hydrazide linker group result in optimal antibody titers against ovine submaxillary mucin and sialyl-Tn-positive tumor cells.Cancer Immunol Immunother. 1999 Apr;48(1):1-8. doi: 10.1007/s002620050542. Cancer Immunol Immunother. 1999. PMID: 10235483 Free PMC article.
-
Synthesis of sialyl Lewis(a) (sLe (a), CA19-9) and construction of an immunogenic sLe(a) vaccine.Cancer Immunol Immunother. 2009 Sep;58(9):1397-405. doi: 10.1007/s00262-008-0654-7. Epub 2009 Feb 4. Cancer Immunol Immunother. 2009. PMID: 19190907 Free PMC article.
-
Cancer vaccines: an update with special focus on ganglioside antigens.Oncol Rep. 2002 Mar-Apr;9(2):267-76. Oncol Rep. 2002. PMID: 11836591 Review.
-
Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer.Clin Breast Cancer. 2003 Feb;3 Suppl 4:S134-8. doi: 10.3816/cbc.2003.s.002. Clin Breast Cancer. 2003. PMID: 12620150 Review.
Cited by
-
Carbohydrate-based cancer vaccines: target cancer with sugar bullets.Glycoconj J. 2012 Aug;29(5-6):259-71. doi: 10.1007/s10719-012-9399-9. Epub 2012 Jun 6. Glycoconj J. 2012. PMID: 22669462 Review.
-
A cell-free biosynthesis platform for modular construction of protein glycosylation pathways.Nat Commun. 2019 Nov 27;10(1):5404. doi: 10.1038/s41467-019-12024-9. Nat Commun. 2019. PMID: 31776339 Free PMC article.
-
Synthesis and evaluation of monophosphoryl lipid A derivatives as fully synthetic self-adjuvanting glycoconjugate cancer vaccine carriers.Org Biomol Chem. 2014 May 28;12(20):3238-45. doi: 10.1039/c4ob00390j. Epub 2014 Apr 14. Org Biomol Chem. 2014. PMID: 24728423 Free PMC article.
-
Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-D-mannosamine.Biochemistry. 2006 Mar 21;45(11):3733-9. doi: 10.1021/bi052161r. Biochemistry. 2006. PMID: 16533056 Free PMC article.
-
Synthetic Glycobiology: Parts, Systems, and Applications.ACS Synth Biol. 2020 Jul 17;9(7):1534-1562. doi: 10.1021/acssynbio.0c00210. Epub 2020 Jun 30. ACS Synth Biol. 2020. PMID: 32526139 Free PMC article. Review.
References
-
- Hakomori S. Possible functions of tumor-associated carbohydrate antigens. Curr Opin Immunol. 1991;3:646–653. - PubMed
-
- Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;3:97–104. - PubMed
-
- Reisfeld RA, Cheresh DAH. Human tumor antigens. Adv Immunol. 1987;40:323–377. - PubMed
-
- Ben-Efraim S. One hundred years of cancer immunotherapy: a critical appraisal. Tumor Biol. 1999;20:1–24. - PubMed
-
- Ragupathi G. Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol Immunother. 1998;46:82–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

