Human transthyretin in complex with iododiflunisal: structural features associated with a potent amyloid inhibitor
- PMID: 15689188
- PMCID: PMC1138969
- DOI: 10.1042/BJ20042035
Human transthyretin in complex with iododiflunisal: structural features associated with a potent amyloid inhibitor
Abstract
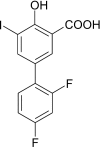
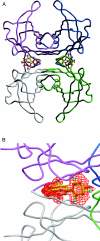
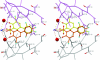
Ex vivo and in vitro studies have revealed the remarkable amyloid inhibitory potency and specificity of iododiflunisal in relation to transthyretin [Almeida, Macedo, Cardoso, Alves, Valencia, Arsequell, Planas and Saraiva (2004) Biochem. J. 381, 351-356], a protein implicated in familial amyloidotic polyneuropathy. In the present paper, the crystal structure of transthyretin complexed with this diflunisal derivative is reported, which enables a detailed analysis of the protein-ligand interactions. Iododiflunisal binds very deep in the hormone-binding channel. The iodine substituent is tightly anchored into a pocket of the binding site and the fluorine atoms provide extra hydrophobic contacts with the protein. The carboxylate substituent is involved in an electrostatic interaction with the N(zeta) of a lysine residue. Moreover, ligand-induced conformational alterations in the side chain of some residues result in the formation of new intersubunit hydrogen bonds. All these new interactions, induced by iododiflunisal, increase the stability of the tetramer impairing the formation of amyloid fibrils. The crystal structure of this complex opens perspectives for the design of more specific and effective drugs for familial amyloidotic polyneuropathy patients.
Figures

Similar articles
-
Selective binding to transthyretin and tetramer stabilization in serum from patients with familial amyloidotic polyneuropathy by an iodinated diflunisal derivative.Biochem J. 2004 Jul 15;381(Pt 2):351-6. doi: 10.1042/BJ20040011. Biochem J. 2004. PMID: 15080795 Free PMC article.
-
Repurposing Benzbromarone for Familial Amyloid Polyneuropathy: A New Transthyretin Tetramer Stabilizer.Int J Mol Sci. 2020 Sep 28;21(19):7166. doi: 10.3390/ijms21197166. Int J Mol Sci. 2020. PMID: 32998442 Free PMC article.
-
Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis.J Med Chem. 2004 Jan 15;47(2):355-74. doi: 10.1021/jm030347n. J Med Chem. 2004. PMID: 14711308
-
Amyloid disease prevention by transthyretin native state complexation with carborane derivatives lacking cyclooxygenase inhibition.Drug News Perspect. 2008 Jun;21(5):258-66. doi: 10.1358/dnp.2008.21.5.1219011. Drug News Perspect. 2008. PMID: 18596990 Review.
-
Conformational change and assembly through edge beta strands in transthyretin and other amyloid proteins.Acc Chem Res. 2006 Sep;39(9):576-83. doi: 10.1021/ar050017s. Acc Chem Res. 2006. PMID: 16981673 Review.
Cited by
-
Diphenyl-Methane Based Thyromimetic Inhibitors for Transthyretin Amyloidosis.Int J Mol Sci. 2021 Mar 28;22(7):3488. doi: 10.3390/ijms22073488. Int J Mol Sci. 2021. PMID: 33800546 Free PMC article.
-
Modifications of the 7-Hydroxyl Group of the Transthyretin Ligand Luteolin Provide Mechanistic Insights into Its Binding Properties and High Plasma Specificity.PLoS One. 2016 Apr 6;11(4):e0153112. doi: 10.1371/journal.pone.0153112. eCollection 2016. PLoS One. 2016. PMID: 27050398 Free PMC article.
-
Rational Design, Synthesis, Characterization and Evaluation of Iodinated 4,4'-Bipyridines as New Transthyretin Fibrillogenesis Inhibitors.Molecules. 2020 May 8;25(9):2213. doi: 10.3390/molecules25092213. Molecules. 2020. PMID: 32397334 Free PMC article.
-
Calorimetric Studies of Binary and Ternary Molecular Interactions between Transthyretin, Aβ Peptides, and Small-Molecule Chaperones toward an Alternative Strategy for Alzheimer's Disease Drug Discovery.J Med Chem. 2020 Mar 26;63(6):3205-3214. doi: 10.1021/acs.jmedchem.9b01970. Epub 2020 Mar 18. J Med Chem. 2020. PMID: 32124607 Free PMC article.
-
Discovery of Bispecific Antagonists of Retinol Binding Protein 4 That Stabilize Transthyretin Tetramers: Scaffolding Hopping, Optimization, and Preclinical Pharmacological Evaluation as a Potential Therapy for Two Common Age-Related Comorbidities.J Med Chem. 2020 Oct 8;63(19):11054-11084. doi: 10.1021/acs.jmedchem.0c00996. Epub 2020 Sep 17. J Med Chem. 2020. PMID: 32878437 Free PMC article.
References
-
- Blake C. C., Geisow M. J., Oatley S. J., Rerat B., Rerat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 Å. J. Mol. Biol. 1978;121:339–356. - PubMed
-
- Serpell L. C., Fraser P. E., Sunde M. X-ray fiber diffraction of amyloid fibrils. Methods Enzymol. 1999;309:526–536. - PubMed
-
- Olofsson A., Ippel H. J., Baranov V., Horstedt P., Wijmenga S., Lundgren E. Capture of a dimeric intermediate during transthyretin amyloid formation. J. Biol. Chem. 2001;276:39592–39599. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

