Dysregulation of Na+/K+ ATPase by amyloid in APP+PS1 transgenic mice
- PMID: 15689237
- PMCID: PMC549198
- DOI: 10.1186/1471-2202-6-7
Dysregulation of Na+/K+ ATPase by amyloid in APP+PS1 transgenic mice
Abstract
Background: The pathology of Alzheimer's disease (AD) is comprised of extracellular amyloid plaques, intracellular tau tangles, dystrophic neurites and neurodegeneration. The mechanisms by which these various pathological features arise are under intense investigation. Here, expanding upon pilot gene expression studies, we have further analyzed the relationship between Na+/K+ ATPase and amyloid using APP+PS1 transgenic mice, a model that develops amyloid plaques and memory deficits in the absence of tangle formation and neuronal or synaptic loss.
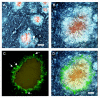
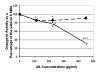
Results: We report that in addition to decreased mRNA expression, there was decreased overall Na+/K+ ATPase enzyme activity in the amyloid-containing hippocampi of the APP+PS1 mice (although not in the amyloid-free cerebellum). In addition, dual immunolabeling revealed an absence of Na+/K+ ATPase staining in a zone surrounding congophilic plaques that was occupied by dystrophic neurites. We also demonstrate that cerebral Na+/K+ ATPase activity can be directly inhibited by high concentrations of soluble Abeta.
Conclusions: The data suggest that the reductions in Na+/K+ ATPase activity in Alzheimer tissue may not be purely secondary to neuronal loss, but may results from direct effects of amyloid on this enzyme. This disruption of ion homeostasis and osmotic balance may interfere with normal electrotonic properties of dendrites, blocking intraneuronal signal processing, and contribute to neuritic dystrophia. These results suggest that therapies aimed at enhancing Na+/K+ ATPase activity in AD may improve symptoms and/or delay disease progression.
Figures



Similar articles
-
Neuropathology of mice carrying mutant APP(swe) and/or PS1(M146L) transgenes: alterations in the p75(NTR) cholinergic basal forebrain septohippocampal pathway.Exp Neurol. 2001 Aug;170(2):227-43. doi: 10.1006/exnr.2001.7710. Exp Neurol. 2001. PMID: 11476589
-
Amyloid suppresses induction of genes critical for memory consolidation in APP + PS1 transgenic mice.J Neurochem. 2004 Jan;88(2):434-42. doi: 10.1111/j.1471-4159.2004.02185.x. J Neurochem. 2004. PMID: 14690531
-
Treadmill exercise enhances synaptic plasticity, but does not alter β-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice.Neuroscience. 2015 Jul 9;298:357-66. doi: 10.1016/j.neuroscience.2015.04.038. Epub 2015 Apr 23. Neuroscience. 2015. PMID: 25917310
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Cell biology and dynamics of Neuronal Na+/K+-ATPase in health and diseases.Neuropharmacology. 2020 Jun 1;169:107461. doi: 10.1016/j.neuropharm.2018.12.008. Epub 2018 Dec 11. Neuropharmacology. 2020. PMID: 30550795 Review.
Cited by
-
S-Adenosylmethionine Promotes Oxidative Stress and Decreases Na+, K+-ATPase Activity in Cerebral Cortex Supernatants of Adolescent Rats: Implications for the Pathogenesis of S-Adenosylhomocysteine Hydrolase Deficiency.Mol Neurobiol. 2018 Jul;55(7):5868-5878. doi: 10.1007/s12035-017-0804-z. Epub 2017 Nov 3. Mol Neurobiol. 2018. PMID: 29101646
-
Amyloid, hyperactivity, and metabolism: theoretical comment on Vloeberghs et al. (2008).Behav Neurosci. 2008 Jun;122(3):730-2. doi: 10.1037/0735-7044.122.3.730. Behav Neurosci. 2008. PMID: 18513144 Free PMC article.
-
Direct interaction of beta-amyloid with Na,K-ATPase as a putative regulator of the enzyme function.Sci Rep. 2016 Jun 14;6:27738. doi: 10.1038/srep27738. Sci Rep. 2016. PMID: 27296892 Free PMC article.
-
Glial Na(+) -dependent ion transporters in pathophysiological conditions.Glia. 2016 Oct;64(10):1677-97. doi: 10.1002/glia.23030. Epub 2016 Jul 26. Glia. 2016. PMID: 27458821 Free PMC article. Review.
-
The Influence of Na(+), K(+)-ATPase on Glutamate Signaling in Neurodegenerative Diseases and Senescence.Front Physiol. 2016 Jun 2;7:195. doi: 10.3389/fphys.2016.00195. eCollection 2016. Front Physiol. 2016. PMID: 27313535 Free PMC article. Review.
References
-
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. - DOI - PubMed
-
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

