Meiotic S-phase damage activates recombination without checkpoint arrest
- PMID: 15689488
- PMCID: PMC1073649
- DOI: 10.1091/mbc.e04-10-0934
Meiotic S-phase damage activates recombination without checkpoint arrest
Abstract
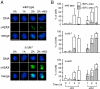
Checkpoints operate during meiosis to ensure the completion of DNA synthesis and programmed recombination before the initiation of meiotic divisions. Studies in the fission yeast Schizosaccharomyces pombe suggest that the meiotic response to DNA damage due to a failed replication checkpoint response differs substantially from the vegetative response, and may be influenced by the presence of homologous chromosomes. The checkpoint responses to DNA damage during fission yeast meiosis are not well characterized. Here we report that DNA damage induced during meiotic S-phase does not activate checkpoint arrest. We also find that in wild-type cells, markers for DNA breaks can persist at least to the first meiotic division. We also observe increased spontaneous S-phase damage in checkpoint mutants, which is repaired by recombination without activating checkpoint arrest. Our results suggest that fission yeast meiosis is exceptionally tolerant of DNA damage, and that some forms of spontaneous S-phase damage can be repaired by recombination without activating checkpoint arrest.
Figures


Similar articles
-
Rad3-Cds1 mediates coupling of initiation of meiotic recombination with DNA replication. Mei4-dependent transcription as a potential target of meiotic checkpoint.J Biol Chem. 2006 Jan 20;281(3):1338-44. doi: 10.1074/jbc.M505767200. Epub 2005 Nov 14. J Biol Chem. 2006. PMID: 16286472
-
Mitotic replication initiation proteins are not required for pre-meiotic S phase.Nat Genet. 2000 Jul;25(3):263-8. doi: 10.1038/77015. Nat Genet. 2000. PMID: 10888871
-
The fission yeast meiotic checkpoint kinase Mek1 regulates nuclear localization of Cdc25 by phosphorylation.Cell Cycle. 2008 Dec;7(23):3720-30. doi: 10.4161/cc.7.23.7177. Epub 2008 Dec 13. Cell Cycle. 2008. PMID: 19029820
-
DNA structure dependent checkpoints as regulators of DNA repair.DNA Repair (Amst). 2002 Dec 5;1(12):983-94. doi: 10.1016/s1568-7864(02)00165-9. DNA Repair (Amst). 2002. PMID: 12531008 Review.
-
Regulation of chromosome dynamics by Hsk1/Cdc7 kinase.Biochem Soc Trans. 2013 Dec;41(6):1712-9. doi: 10.1042/BST20130217. Biochem Soc Trans. 2013. PMID: 24256280 Review.
Cited by
-
Meiotic chromosome mobility in fission yeast is resistant to environmental stress.Sci Rep. 2016 Apr 14;6:24222. doi: 10.1038/srep24222. Sci Rep. 2016. PMID: 27074839 Free PMC article.
-
CDK contribution to DSB formation and recombination in fission yeast meiosis.PLoS Genet. 2019 Jan 14;15(1):e1007876. doi: 10.1371/journal.pgen.1007876. eCollection 2019 Jan. PLoS Genet. 2019. PMID: 30640914 Free PMC article.
-
Replication fork stability is essential for the maintenance of centromere integrity in the absence of heterochromatin.Cell Rep. 2013 Mar 28;3(3):638-45. doi: 10.1016/j.celrep.2013.02.007. Epub 2013 Mar 7. Cell Rep. 2013. PMID: 23478021 Free PMC article.
-
Activation of an alternative, rec12 (spo11)-independent pathway of fission yeast meiotic recombination in the absence of a DNA flap endonuclease.Genetics. 2005 Dec;171(4):1499-511. doi: 10.1534/genetics.105.046821. Epub 2005 Aug 22. Genetics. 2005. PMID: 16118186 Free PMC article.
-
Schizosaccharomyces pombe histone acetyltransferase Mst1 (KAT5) is an essential protein required for damage response and chromosome segregation.Genetics. 2008 Jun;179(2):757-71. doi: 10.1534/genetics.107.085779. Epub 2008 May 27. Genetics. 2008. PMID: 18505873 Free PMC article.
References
-
- Barlow, C., Hirotsune, S., Paylor, R., Liyanage, M., Eckhaus, M., Collins, F., Shiloh, Y., Crawley, J. N., Ried, T., Tagle, D., and Wynshaw-Boris, A. (1996). Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171. - PubMed
-
- Barlow, C., Liyanage, M., Moens, P. B., Tarsounas, M., Nagashima, K., Brown, K., Rottinghaus, S., Jackson, S. P., Tagle, D., Ried, T., and Wynshaw-Boris, A. (1998). Atm deficiency results in severe meiotic disruption as early as leptonemia of prophase I. Development 125, 4007–4017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

