Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies
- PMID: 15689493
- PMCID: PMC1073686
- DOI: 10.1091/mbc.e04-08-0737
Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies
Abstract
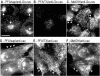
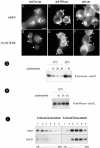
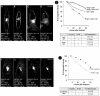
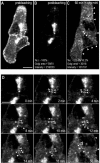
Caveolins are a crucial component of plasma membrane (PM) caveolae but have also been localized to intracellular compartments, including the Golgi complex and lipid bodies. Mutant caveolins associated with human disease show aberrant trafficking to the PM and Golgi accumulation. We now show that the Golgi pool of mainly newly synthesized protein is detergent-soluble and predominantly in a monomeric state, in contrast to the surface pool. Caveolin at the PM is not recognized by specific caveolin antibodies unless PM cholesterol is depleted. Exit from the Golgi complex of wild-type caveolin-1 or -3, but not vesicular stomatitis virus-G protein, is modulated by changing cellular cholesterol levels. In contrast, a muscular dystrophy-associated mutant of caveolin-3, Cav3P104L, showed increased accumulation in the Golgi complex upon cholesterol treatment. In addition, we demonstrate that in response to fatty acid treatment caveolin can follow a previously undescribed pathway from the PM to lipid bodies and can move from lipid bodies to the PM in response to removal of fatty acids. The results suggest that cholesterol is a rate-limiting component for caveolin trafficking. Changes in caveolin flux through the exocytic pathway can therefore be an indicator of cellular cholesterol and fatty acid levels.
Figures






Similar articles
-
Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation.J Cell Biol. 1994 Dec;127(5):1185-97. doi: 10.1083/jcb.127.5.1185. J Cell Biol. 1994. PMID: 7962084 Free PMC article.
-
Molecular characterization of caveolin association with the Golgi complex: identification of a cis-Golgi targeting domain in the caveolin molecule.J Cell Biol. 1999 Jun 28;145(7):1443-59. doi: 10.1083/jcb.145.7.1443. J Cell Biol. 1999. PMID: 10385524 Free PMC article.
-
Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant.Mol Biol Cell. 2004 Jan;15(1):99-110. doi: 10.1091/mbc.e03-06-0368. Epub 2003 Oct 3. Mol Biol Cell. 2004. PMID: 14528016 Free PMC article.
-
The caveolin triad: caveolae biogenesis, cholesterol trafficking, and signal transduction.Cytokine Growth Factor Rev. 2001 Mar;12(1):41-51. doi: 10.1016/s1359-6101(00)00022-8. Cytokine Growth Factor Rev. 2001. PMID: 11312118 Review.
-
Intracellular cholesterol transport.J Lipid Res. 1997 Aug;38(8):1503-21. J Lipid Res. 1997. PMID: 9300773 Review.
Cited by
-
Overexpression of caveolin-1 is sufficient to phenocopy the behavior of a disease-associated mutant.Traffic. 2013 Jun;14(6):663-77. doi: 10.1111/tra.12066. Epub 2013 Apr 7. Traffic. 2013. PMID: 23469926 Free PMC article.
-
MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes.J Cell Biol. 2009 Jun 29;185(7):1259-73. doi: 10.1083/jcb.200903053. Epub 2009 Jun 22. J Cell Biol. 2009. PMID: 19546242 Free PMC article.
-
Cholesterol effectively blocks entry of flavivirus.J Virol. 2008 Jul;82(13):6470-80. doi: 10.1128/JVI.00117-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448543 Free PMC article.
-
Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains.J Cell Biol. 2013 Dec 23;203(6):985-1001. doi: 10.1083/jcb.201305142. J Cell Biol. 2013. PMID: 24368806 Free PMC article.
-
Prominin-2 expression increases protrusions, decreases caveolae and inhibits Cdc42 dependent fluid phase endocytosis.Biochem Biophys Res Commun. 2013 May 10;434(3):466-72. doi: 10.1016/j.bbrc.2013.03.097. Epub 2013 Apr 10. Biochem Biophys Res Commun. 2013. PMID: 23583380 Free PMC article.
References
-
- Bist, A., Fielding, C. J., and Fielding, P. E. (2000). p53 regulates caveolin gene transcription, cell cholesterol, and growth by a novel mechanism. Biochemistry 39, 1966-1972. - PubMed
-
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254. - PubMed
-
- Dundr, M., Hoffmann-Rohrer, U., Hu, Q., Grummt, I., Rothblum, L. I., Phair, R. D., and Misteli, T. (2002). A kinetic framework for a mammalian RNA polymerase in vivo. Science 298, 1623-1626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

