A Rab requirement is not bypassed in SLY1-20 suppression
- PMID: 15689495
- PMCID: PMC1073665
- DOI: 10.1091/mbc.e04-08-0725
A Rab requirement is not bypassed in SLY1-20 suppression
Abstract
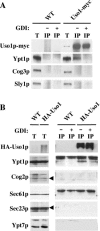
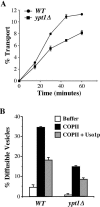
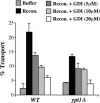
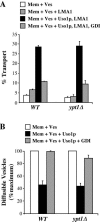
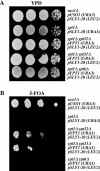
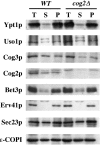
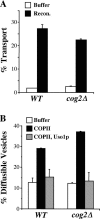
The Rab GTPase Ypt1p and the large homodimer Uso1p are both required for tethering endoplasmic reticulum-derived vesicles to early Golgi compartments in yeast. Loss-of-function ypt1 and uso1 mutations are suppressed by SLY1-20, a dominant allele that encodes the Sed5p-associated protein, Sly1p. Here, we investigate the mechanism of SLY1-20 suppression. In wild-type strains, Ypt1p can be coimmunoprecipitated with Uso1p; however, in a ypt1Delta/SLY1-20 strain, which lacks this complex, membrane binding of Uso1p was reduced. In spite of Ypt1p depletion, Uso1p-dependent vesicle tethering was not bypassed under the ypt1Delta/SLY1-20 condition. Moreover, tethering and fusion assays with ypt1Delta/SLY1-20 membranes remained sensitive to Rab GDP dissociation inhibitor. These results indicate that an alternative Rab protein satisfies the Ypt1p requirement in Uso1p-dependent tethering when SLY1-20 is expressed. Further genetic and biochemical tests revealed that a related Rab protein, Ypt6, might substitute for Ypt1p in ypt1Delta/SLY1-20 cells. Additional experimentation to address the mechanism of SLY1-20 suppression in a cog2Delta [sec35Delta] strain indicated that the Cog2p subunit of the conserved oligomeric Golgi complex is either functionally redundant or is not directly required for anterograde transport to the Golgi complex.
Figures



Similar articles
-
Assembly of the ER to Golgi SNARE complex requires Uso1p.J Cell Biol. 1996 Mar;132(5):755-67. doi: 10.1083/jcb.132.5.755. J Cell Biol. 1996. PMID: 8603910 Free PMC article.
-
Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins.EMBO J. 1998 Apr 15;17(8):2156-65. doi: 10.1093/emboj/17.8.2156. EMBO J. 1998. PMID: 9545229 Free PMC article.
-
Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes.J Cell Biol. 2000 Apr 3;149(1):55-66. doi: 10.1083/jcb.149.1.55. J Cell Biol. 2000. PMID: 10747087 Free PMC article.
-
Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking.J Cell Biol. 1998 Jun 1;141(5):1107-19. doi: 10.1083/jcb.141.5.1107. J Cell Biol. 1998. PMID: 9606204 Free PMC article.
-
[Rab GTPases networks in membrane traffic in Saccharomyces cerevisiae].Yakugaku Zasshi. 2015;135(3):483-92. doi: 10.1248/yakushi.14-00246. Yakugaku Zasshi. 2015. PMID: 25759056 Review. Japanese.
Cited by
-
The Golgin protein Coy1 functions in intra-Golgi retrograde transport and interacts with the COG complex and Golgi SNAREs.Mol Biol Cell. 2017 Aug 9;28(20):2686-700. doi: 10.1091/mbc.E17-03-0137. Online ahead of print. Mol Biol Cell. 2017. PMID: 28794270 Free PMC article.
-
The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association.J Biol Chem. 2009 Jun 12;284(24):16118-16125. doi: 10.1074/jbc.M109.000737. Epub 2009 Apr 21. J Biol Chem. 2009. PMID: 19386605 Free PMC article.
-
Multicopy suppressor analysis of thermosensitive YIP1 alleles implicates GOT1 in transport from the ER.J Cell Sci. 2009 May 15;122(Pt 10):1540-50. doi: 10.1242/jcs.042457. Epub 2009 Apr 21. J Cell Sci. 2009. PMID: 19383723 Free PMC article.
-
On and off membrane dynamics of the endoplasmic reticulum-golgi tethering factor p115 in vivo.Mol Biol Cell. 2006 Jul;17(7):2996-3008. doi: 10.1091/mbc.e05-09-0862. Epub 2006 Apr 19. Mol Biol Cell. 2006. PMID: 16624868 Free PMC article.
-
Exploring Novel Functions of the Small GTPase Ypt1p under Heat-Shock by Characterizing a Temperature-Sensitive Mutant Yeast Strain, ypt1-G80D.Int J Mol Sci. 2019 Jan 1;20(1):132. doi: 10.3390/ijms20010132. Int J Mol Sci. 2019. PMID: 30609659 Free PMC article.
References
-
- Allan, B. B., Moyer, B. D., and Balch, W. E. (2000). Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444–448. - PubMed
-
- Araki, S., Kikuchi, A., Hata, Y., Isomura, M., and Takai, Y. (1990). Regulation of reversible binding of smg p25A, a ras p21-like GTP binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J. Biol. Chem. 265, 13007–13015. - PubMed
-
- Baker, D., Hicke, L., Rexach, M., Schleyer, M., and Schekman, R. (1988). Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell 54, 335–344. - PubMed
-
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, D., Hamamoto, S., Salama, N., Rexach, M., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the ER. Cell 77, 895–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

