Beta2-microglobulin isoforms display an heterogeneous affinity for type I collagen
- PMID: 15689502
- PMCID: PMC2279294
- DOI: 10.1110/ps.041194005
Beta2-microglobulin isoforms display an heterogeneous affinity for type I collagen
Abstract
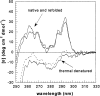
It has been claimed that beta2-microglobulin (beta2-m) interacts with type I and type II collagen, and this property has been linked to the tissue specificity of the beta2-m amyloid deposits that target the osteo-articular system. The binding parameters of the interaction between collagen and beta2-m were determined by band shift electrophoresis and surface plasma resonance by using bovine collagen of type I and type II and various isoforms of beta2-m. Wild-type beta2-m binds collagen type I with a Kd of 4.1 x 10(-4) M and type II with 2.3 x 10(-3) M. By the BIAcore system we monitored the binding properties of the conformers of the slow phase of folding of beta2-m. The folding intermediates during the slow phase of folding do not display any significant difference with respect to the binding properties of the fully folded molecule. The affinity of beta2-m truncated at the third N-terminal residue does not differ from that reported for the wild-type protein. Increased affinity for collagen type I is found in the case of N-terminal truncated species lacking of six residues. The Kd of this species is 3.4 x 10 (-5) M at pH 7.4 and its affinity increases to 4.9 x 10(-6) M at pH 6.4. Fluctuations of the affinity caused by beta2-m truncation and pH change can cause modifications of protein concentration in the solvent that surrounds the collagen, and could contribute to generate locally a critical protein concentration able to prime the protein aggregation.
Figures

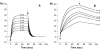

References
-
- Antosiewicz, J., McCammon, J.A., and Gilson, M.K. 1994. Prediction of pH-dependent properties of proteins. J. Mol. Biol. 238 415–436. - PubMed
-
- Bellotti, V., Stoppini, M., Mangione, P., Sunde, M., Robinson, C., Asti, L., Brancaccio, D., and Ferri, G. 1998. β2-microglobulin can be refolded into a native state from ex vivo amyloid fibrils. Eur. J. Biochem. 258 61–67. - PubMed
-
- Chiti, F., Mangione, P., Andreola, A., Giorgetti, S., Stefani, M., Dobson, C.M., Bellotti, V., and Taddei, N. 2001a. Detection of two partially structured species in the folding process of the amyloidogenic protein beta; 2-microglobulin. J. Mol. Biol. 307 379–391. - PubMed
-
- Chiti, F., De Lorenzi, E., Grossi, S., Mangione, P., Giorgetti, S., Caccialanza, G., Dobson, C.M., Merlini, G., Ramponi, G., and Bellotti, V. 2001b. A partially structured species of beta;2-microglobulin is significantly populated under physiological conditions and involved in fibrillogenesis. J. Biol. Chem. 276 46714–46721. - PubMed
-
- Corazza, A., Pettirossi, F., Viglino, P., Verdone, G., Garcia, J., Dumy, P., Giorgetti, S., Mangione, P., Raimondi, S., Stoppini, M., et al. 2004. Properties of some variants of human beta;2-microglobulin and amyloidogenesis. J. Biol. Chem. 279 9176–9189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

