Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation
- PMID: 15689566
- PMCID: PMC6725957
- DOI: 10.1523/JNEUROSCI.4086-04.2005
Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation
Abstract
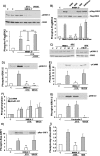
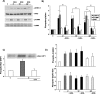
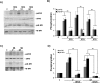
Intracellular Ca2+ and protein phosphorylation play pivotal roles in long-term potentiation (LTP), a cellular model of learning and memory. Ca2+ regulates multiple intracellular pathways, including the calmodulin-dependent kinases (CaMKs) and the ERKs (extracellular signal-regulated kinases), both of which are required for LTP. However, the mechanism by which Ca2+ activates ERK during LTP remains unknown. Here, we describe a requirement for the CaMK-kinase (CaMKK) pathway upstream of ERK in LTP induction. Both the pharmacological inhibitor of CaMKK, STO-609, and dominant-negative CaMKI (dnCaMKI), a downstream target of CaMKK, blocked neuronal NMDA receptor-dependent ERK activation. In contrast, an inhibitor of CaMKII and nuclear-localized dnCaMKIV had no effect on ERK activation. NMDA receptor-dependent LTP induction robustly activated CaMKI, the Ca2+-stimulated Ras activator Ras-GRF1 (Ras-guanyl-nucleotide releasing factor), and ERK. STO-609 blocked the activation of all three enzymes during LTP without affecting basal synaptic transmission, activation of CaMKII, or cAMP-dependent activation of ERK. LTP induction itself was suppressed 50% by STO-609 in a manner identical to the ERK inhibitor U0126: either inhibitor occluded the effect of the other, suggesting they are part of the same signaling pathway in LTP induction. STO-609 also suppressed regulatory phosphorylation of two downstream ERK targets during LTP, the general translation factors eIF4E (eukaryotic initiation factor 4) and its binding protein 4E-BP1 (eukaryotic initiation factor 4E-binding protein 1). These data indicate an essential role for CaMKK and CaMKI to link NMDA receptor-mediated Ca2+ elevation with ERK-dependent LTP.
Figures



References
-
- Agell N, Bachs O, Rocamora N, Villalonga P (2002) Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+ and calmodulin. Cell Signal 14: 649-654. - PubMed
-
- Asaki C, Usuda N, Nakazawa A, Kametani K, Suzuki T (2003) Localization of translational components at the ultramicroscopic level at postsynaptic sites of the rat brain. Brain Res 972: 168-176. - PubMed
-
- Baouz S, Jacquet E, Accorsi K, Hountondji C, Balestrini M, Zippel R, Sturani E, Parmeggiani A (2001) Sites of phosphorylation by protein kinase A in CDC25Mm/GRF1, a guanine nucleotide exchange factor for Ras. J Biol Chem 276: 1742-1749. - PubMed
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR (1997) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276: 2042-2045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
