Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization
- PMID: 15690082
- PMCID: PMC546422
- DOI: 10.1172/JCI22681
Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization
Abstract
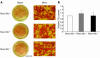
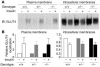
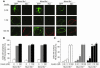
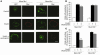
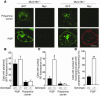
Insulin-stimulated glucose uptake in adipocytes is mediated by translocation of vesicles containing the glucose transporter GLUT4 from intracellular storage sites to the cell periphery and the subsequent fusion of these vesicles with the plasma membrane, resulting in the externalization of GLUT4. Fusion of the GLUT4-containing vesicles with the plasma membrane is mediated by a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex consisting of vesicle-associated membrane protein 2 (VAMP2), 23-kDa synaptosomal-associated protein (SNAP23), and syntaxin4. We have now generated mouse embryos deficient in the syntaxin4 binding protein Munc18c and show that the insulin-induced appearance of GLUT4 at the cell surface is enhanced in adipocytes derived from these Munc18c-/- mice compared with that in Munc18c+/+ cells. Wortmannin, an inhibitor of PI3K, inhibited insulin-stimulated GLUT4 externalization, without affecting GLUT4 translocation to the cell periphery, in Munc18c+/+ adipocytes, but it did not affect GLUT4 externalization in Munc18c-/- cells. Phosphatidylinositol 3-phosphate, which induced GLUT4 translocation to the cell periphery without externalization in Munc18c+/+ cells, elicited GLUT4 externalization in Munc18c-/- cells. These findings demonstrate that Munc18c inhibits insulin-stimulated externalization of GLUT4 in a wortmannin-sensitive manner, and they suggest that disruption of the interaction between syntaxin4 and Munc18c in adipocytes might result in enhancement of insulin-stimulated GLUT4 externalization.
Figures



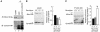

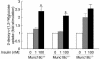

Comment in
- J Clin Invest. 115:219.
References
-
- Holman GD, Sandoval IV. Moving the insulin-regulated glucose transporter GLUT4 into and out of storage. Trends Cell Biol. 2001;11:173–179. - PubMed
-
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002;3:267–277. - PubMed
-
- Araki S, et al. Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c. Biochem. Biophys. Res. Commun. 1997;234:257–262. - PubMed
-
- Tellam JT, et al. Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. J. Biol. Chem. 1997;272:6179–6186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

