SOCS2 negatively regulates growth hormone action in vitro and in vivo
- PMID: 15690087
- PMCID: PMC546423
- DOI: 10.1172/JCI22710
SOCS2 negatively regulates growth hormone action in vitro and in vivo
Abstract
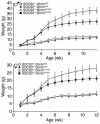
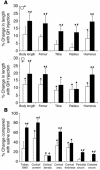
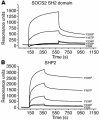
Mice deficient in SOCS2 display an excessive growth phenotype characterized by a 30-50% increase in mature body size. Here we show that the SOCS2-/- phenotype is dependent upon the presence of endogenous growth hormone (GH) and that treatment with exogenous GH induced excessive growth in mice lacking both endogenous GH and SOCS2. This was reflected in terms of overall body weight, body and bone lengths, and the weight of internal organs and tissues. A heightened response to GH was also measured by examining GH-responsive genes expressed in the liver after exogenous GH administration. To further understand the link between SOCS2 and the GH-signaling cascade, we investigated the nature of these interactions using structure/function and biochemical interaction studies. Analysis of the 3 structural motifs of the SOCS2 molecule revealed that each plays a crucial role in SOCS2 function, with the conserved SOCS-box motif being essential for all inhibitory function. SOCS2 was found to bind 2 phosphorylated tyrosines on the GH receptor, and mutational analysis of these amino acids showed that both were essential for SOCS2 function. Together, the data provide clear evidence that SOCS2 is a negative regulator of GH signaling.
Figures

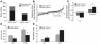

Comment in
-
Knock your SOCS off!J Clin Invest. 2005 Feb;115(2):233-6. doi: 10.1172/JCI24228. J Clin Invest. 2005. PMID: 15690080 Free PMC article. Review.
Similar articles
-
Knock your SOCS off!J Clin Invest. 2005 Feb;115(2):233-6. doi: 10.1172/JCI24228. J Clin Invest. 2005. PMID: 15690080 Free PMC article. Review.
-
Suppressor of cytokine signaling-2 deficiency induces molecular and metabolic changes that partially overlap with growth hormone-dependent effects.Mol Endocrinol. 2005 Mar;19(3):781-93. doi: 10.1210/me.2004-0040. Epub 2004 Nov 24. Mol Endocrinol. 2005. PMID: 15563548
-
Suppressors of cytokine signalling and regulation of growth hormone action.Growth Horm IGF Res. 2004 Jun;14(3):200-6. doi: 10.1016/j.ghir.2003.12.011. Growth Horm IGF Res. 2004. PMID: 15125881 Review.
-
SOCS2 induces neurite outgrowth by regulation of epidermal growth factor receptor activation.J Biol Chem. 2004 Apr 16;279(16):16349-55. doi: 10.1074/jbc.M312873200. Epub 2004 Feb 4. J Biol Chem. 2004. PMID: 14764607
-
Growth hormone (GH)-stimulated insulin-like growth factor I gene expression is mediated by a tyrosine phosphorylation pathway depending on C-terminal region of human GH receptor in human GH receptor-expressing Ba/F3 cells.Endocrinology. 2004 Jan;145(1):214-20. doi: 10.1210/en.2003-0811. Epub 2003 Oct 9. Endocrinology. 2004. PMID: 14551225
Cited by
-
Suppression of cytokine signaling: the SOCS perspective.Cytokine Growth Factor Rev. 2013 Jun;24(3):241-8. doi: 10.1016/j.cytogfr.2013.03.005. Epub 2013 Mar 30. Cytokine Growth Factor Rev. 2013. PMID: 23545160 Free PMC article. Review.
-
The ElonginB/C-Cullin5-SOCS-Box-Complex Is a Potential Biomarker for Growth Hormone Disorders.Biomedicines. 2021 Feb 17;9(2):201. doi: 10.3390/biomedicines9020201. Biomedicines. 2021. PMID: 33671326 Free PMC article.
-
t(8;9)(p22;p24)/PCM1-JAK2 activates SOCS2 and SOCS3 via STAT5.PLoS One. 2013;8(1):e53767. doi: 10.1371/journal.pone.0053767. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23372669 Free PMC article.
-
SOCS proteins in regulation of receptor tyrosine kinase signaling.Cell Mol Life Sci. 2014 Sep;71(17):3297-310. doi: 10.1007/s00018-014-1619-y. Epub 2014 Apr 5. Cell Mol Life Sci. 2014. PMID: 24705897 Free PMC article. Review.
-
SOCS2 Balances Metabolic and Restorative Requirements during Liver Regeneration.J Biol Chem. 2016 Feb 12;291(7):3346-58. doi: 10.1074/jbc.M115.703264. Epub 2015 Dec 23. J Biol Chem. 2016. PMID: 26703468 Free PMC article.
References
-
- Press M. Growth hormone and metabolism. Diabetes Metab. Rev. 1988;4:391–414. - PubMed
-
- Sonksen PH, Salomon F, Cuneo R. Metabolic effects of hypopituitarism and acromegaly. Horm. Res. 1991;36:S27–S31. - PubMed
-
- [Anonymous]. Consensus guidelines for the diagnosis and treatment of adults with growth hormone deficiency: summary statement of the growth hormone research society workshop on adult growth hormone deficiency. J. Clin. Endocrinol. Metab. 1998;83:379–381. - PubMed
-
- Monson JP. Long-term experience with GH replacement therapy: efficacy and safety. Eur. J. Endocrinol. 2003;148:S9–S14. - PubMed
-
- Bramnert M, et al. Growth hormone replacement therapy induces resistance by activating the glucose-fatty acid cycle. J. Clin. Endocrinol. Metab. 2003;88:1455–1463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

