Role of Leishmania (Leishmania) chagasi amastigote cysteine protease in intracellular parasite survival: studies by gene disruption and antisense mRNA inhibition
- PMID: 15691375
- PMCID: PMC549197
- DOI: 10.1186/1471-2199-6-3
Role of Leishmania (Leishmania) chagasi amastigote cysteine protease in intracellular parasite survival: studies by gene disruption and antisense mRNA inhibition
Abstract
Background: The parasitic protozoa belonging to Leishmania (L.) donovani complex possess abundant, developmentally regulated cathepsin L-like cysteine proteases. Previously, we have reported the isolation of cysteine protease gene, Ldccys2 from Leishmania (L.) chagasi. Here, we have further characterized this cysteine protease gene and demonstrated its role during infection and survival of Leishmania (L.) chagasi within the U937 macrophage cells.
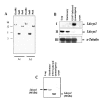
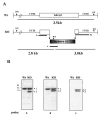
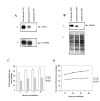
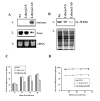
Results: The amastigote specific Ldccys2 genes of L. (L.) chagasi and L. (L.) donovani have identical gene organization, as determined by southern blots. In vivo expression analyses by Northern blots showed that Ldccys2 is amastigote specific. Western blot using anti-Ldccys2 antibody confirmed the amastigote specific protein expression. Recombinant expression of Ldccys2, a 30 kDA protein, was functionally active in a gelatin assay. Results from Ldccys2 heterozygous knockout mutants showed its role during macrophage infection and in intra-macrophage survival of the parasites. Since attempts to generate null mutants failed, we used antisense RNA inhibition to regulate Ldcccys2 gene expression. Not surprisingly, the results from antisense studies further confirmed the results from heterozygous knockout mutants, reiterating the importance of amastigote specific cysteine proteases in Leishmania infection and pathogenesis.
Conclusions: The study shows that Ldccys2 is a developmentally regulated gene and that Ldccys2 is expressed only in infectious amastigote stages of the parasite. The collective results from both the heterozygous knockout mutants and antisense mRNA inhibition studies shows that Ldccys2 helps in infection and survival of L. (L.) chagasi amastigotes within the macrophage cells. Finally, antisense RNA technique can be used as an alternate approach to gene knockout, for silencing gene expression in L. (L.) chagasi, especially in cases such as this, where a null mutant cannot be achieved by homologous recombination.
Figures

Similar articles
-
Molecular cloning, characterization and overexpression of two distinct cysteine protease cDNAs from Leishmania donovani chagasi.Mol Biochem Parasitol. 1997 Dec 1;90(1):247-67. doi: 10.1016/s0166-6851(97)00158-8. Mol Biochem Parasitol. 1997. PMID: 9497047
-
Developmental changes in the expression of Leishmania chagasi gp63 and heat shock protein in a human macrophage cell line.Infect Immun. 1996 May;64(5):1810-8. doi: 10.1128/iai.64.5.1810-1818.1996. Infect Immun. 1996. PMID: 8613395 Free PMC article.
-
Cloning and characterisation of a cysteine proteinase gene expressed in amastigotes of Leishmania (L.) amazonensis.Int J Parasitol. 2003 Apr;33(4):445-54. doi: 10.1016/s0020-7519(03)00010-9. Int J Parasitol. 2003. PMID: 12705937
-
Generation of growth arrested Leishmania amastigotes: a tool to develop live attenuated vaccine candidates against visceral leishmaniasis.Vaccine. 2014 Jun 30;32(31):3895-901. doi: 10.1016/j.vaccine.2014.05.009. Epub 2014 May 14. Vaccine. 2014. PMID: 24837513 Review.
-
Polymorphisms of cpb multicopy genes in the Leishmania (Leishmania) donovani complex.Trans R Soc Trop Med Hyg. 2008 Feb;102(2):105-6. doi: 10.1016/j.trstmh.2007.09.013. Epub 2007 Nov 9. Trans R Soc Trop Med Hyg. 2008. PMID: 17996911 Review.
Cited by
-
Inhibition of Leishmania major PTR1 Gene Expression by Antisense in Escherichia coli.Iran J Public Health. 2012;41(6):65-71. Epub 2012 Jun 30. Iran J Public Health. 2012. PMID: 23113195 Free PMC article.
-
Improving serodiagnosis of human and canine leishmaniasis with recombinant Leishmania braziliensis cathepsin l-like protein and a synthetic peptide containing its linear B-cell epitope.PLoS Negl Trop Dis. 2015 Jan 8;9(1):e3426. doi: 10.1371/journal.pntd.0003426. eCollection 2015 Jan. PLoS Negl Trop Dis. 2015. PMID: 25569432 Free PMC article.
-
Protein turnover and differentiation in Leishmania.Int J Parasitol. 2007 Aug;37(10):1063-75. doi: 10.1016/j.ijpara.2007.03.008. Epub 2007 Mar 31. Int J Parasitol. 2007. PMID: 17493624 Free PMC article. Review.
-
The pathogenicity and virulence of Leishmania - interplay of virulence factors with host defenses.Virulence. 2022 Dec;13(1):903-935. doi: 10.1080/21505594.2022.2074130. Virulence. 2022. PMID: 35531875 Free PMC article. Review.
-
The genetics of Leishmania virulence.Med Microbiol Immunol. 2015 Dec;204(6):619-34. doi: 10.1007/s00430-015-0422-1. Epub 2015 Jun 6. Med Microbiol Immunol. 2015. PMID: 26047933 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

