Nucleus accumbens core lesions retard instrumental learning and performance with delayed reinforcement in the rat
- PMID: 15691387
- PMCID: PMC549214
- DOI: 10.1186/1471-2202-6-9
Nucleus accumbens core lesions retard instrumental learning and performance with delayed reinforcement in the rat
Abstract
Background: Delays between actions and their outcomes severely hinder reinforcement learning systems, but little is known of the neural mechanism by which animals overcome this problem and bridge such delays. The nucleus accumbens core (AcbC), part of the ventral striatum, is required for normal preference for a large, delayed reward over a small, immediate reward (self-controlled choice) in rats, but the reason for this is unclear. We investigated the role of the AcbC in learning a free-operant instrumental response using delayed reinforcement, performance of a previously-learned response for delayed reinforcement, and assessment of the relative magnitudes of two different rewards.
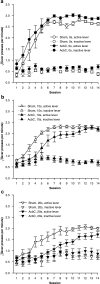
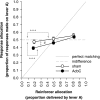
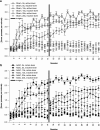
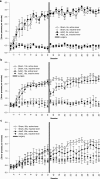
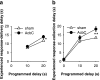
Results: Groups of rats with excitotoxic or sham lesions of the AcbC acquired an instrumental response with different delays (0, 10, or 20 s) between the lever-press response and reinforcer delivery. A second (inactive) lever was also present, but responding on it was never reinforced. As expected, the delays retarded learning in normal rats. AcbC lesions did not hinder learning in the absence of delays, but AcbC-lesioned rats were impaired in learning when there was a delay, relative to sham-operated controls. All groups eventually acquired the response and discriminated the active lever from the inactive lever to some degree. Rats were subsequently trained to discriminate reinforcers of different magnitudes. AcbC-lesioned rats were more sensitive to differences in reinforcer magnitude than sham-operated controls, suggesting that the deficit in self-controlled choice previously observed in such rats was a consequence of reduced preference for delayed rewards relative to immediate rewards, not of reduced preference for large rewards relative to small rewards. AcbC lesions also impaired the performance of a previously-learned instrumental response in a delay-dependent fashion.
Conclusions: These results demonstrate that the AcbC contributes to instrumental learning and performance by bridging delays between subjects' actions and the ensuing outcomes that reinforce behaviour.
Figures

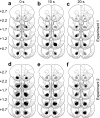
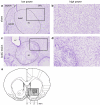
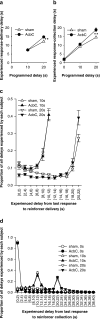
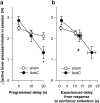
 = .213, p = .004). (d) Distribution of experienced response-collection delays, displayed in the same manner as (c). There were no differences in the distribution of delays experienced by AcbC-lesioned and sham rats in the 0 s condition (lesion and lesion × range, Fs < 1, NS). In the 10 s condition, AcbC-lesioned rats experienced a slightly higher proportion of long response-collection delays and a slightly lower proportion of short response-collection delays (lesion, F1,13 = 6.36, p = .036, though the lesion × range interaction was not significant, F2.6,34.3 = 1.74,
= .213, p = .004). (d) Distribution of experienced response-collection delays, displayed in the same manner as (c). There were no differences in the distribution of delays experienced by AcbC-lesioned and sham rats in the 0 s condition (lesion and lesion × range, Fs < 1, NS). In the 10 s condition, AcbC-lesioned rats experienced a slightly higher proportion of long response-collection delays and a slightly lower proportion of short response-collection delays (lesion, F1,13 = 6.36, p = .036, though the lesion × range interaction was not significant, F2.6,34.3 = 1.74,  = .139, p = .181). Similarly, in the 20 s condition, AcbC-lesioned rats experienced a slightly higher proportion of long response-collection delays and a slightly lower proportion of short response-collection delays than shams (lesion × range, F4.2,54.8 = 6.65,
= .139, p = .181). Similarly, in the 20 s condition, AcbC-lesioned rats experienced a slightly higher proportion of long response-collection delays and a slightly lower proportion of short response-collection delays than shams (lesion × range, F4.2,54.8 = 6.65,  = .222, p < .001).
= .222, p < .001).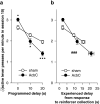
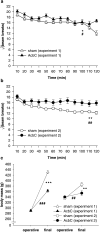
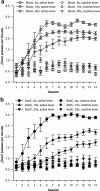
 = .515, p < .001), reflecting habituation, and a lesion × bin interaction (F5.7,237.9 = 2.52,
= .515, p < .001), reflecting habituation, and a lesion × bin interaction (F5.7,237.9 = 2.52,  = .515, # p = .024). (b) Locomotor activity in Experiment 2. The same patterns were observed (data from five subjects were not recorded due to a mechanical error; lesion: F1,37 = 9.155, ** p = .004; bin: F9.3,345.2 = 13.5,
= .515, # p = .024). (b) Locomotor activity in Experiment 2. The same patterns were observed (data from five subjects were not recorded due to a mechanical error; lesion: F1,37 = 9.155, ** p = .004; bin: F9.3,345.2 = 13.5,  = .848, p < .001; lesion × bin: F9.3,345.2 = 3.18,
= .848, p < .001; lesion × bin: F9.3,345.2 = 3.18,  = .848, ## p = .001). (c) Preoperative and final body mass in both experiments. Preoperatively, masses did not differ between groups (Experiment 1: F < 1, NS; Experiment 2: F1,42 = 1.008, NS), but in both cases, AcbC-lesioned subjects gained less mass than controls (Experiment 1: lesion × time: F1,41 = 95.9, ### p < .001; group difference at second time point: F1,42 = 88.4, *** p < .001; Experiment 2: lesion × time: F1,42 = 13.53, ## p = .001; group difference at second time point: F1,42 = 7.37, ** p = .01).
= .848, ## p = .001). (c) Preoperative and final body mass in both experiments. Preoperatively, masses did not differ between groups (Experiment 1: F < 1, NS; Experiment 2: F1,42 = 1.008, NS), but in both cases, AcbC-lesioned subjects gained less mass than controls (Experiment 1: lesion × time: F1,41 = 95.9, ### p < .001; group difference at second time point: F1,42 = 88.4, *** p < .001; Experiment 2: lesion × time: F1,42 = 13.53, ## p = .001; group difference at second time point: F1,42 = 7.37, ** p = .01).References
-
- Dickinson A, Watt A, Griffiths WJH. Free-operant acquisition with delayed reinforcement. Quarterly Journal of Experimental Psychology, Section B - Comparative and Physiological Psychology. 1992;45:241–258.
-
- APA . Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) Washington DC, American Psychiatric Association; 2000.
-
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. - PubMed
-
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

