Improved secretory production of recombinant proteins by random mutagenesis of hlyB, an alpha-hemolysin transporter from Escherichia coli
- PMID: 15691914
- PMCID: PMC546688
- DOI: 10.1128/AEM.71.2.656-662.2005
Improved secretory production of recombinant proteins by random mutagenesis of hlyB, an alpha-hemolysin transporter from Escherichia coli
Abstract
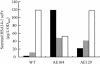
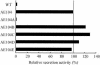
Fusion proteins with an alpha-hemolysin (HlyA) C-terminal signal sequence are known to be secreted by the HlyB-HlyD-TolC translocator in Escherichia coli. We aimed to establish an efficient Hly secretory expression system by random mutagenesis of hlyB and hlyD. The fusion protein of subtilisin E and the HlyA signal sequence (HlyA(218)) was used as a marker protein for evaluating secretion efficiency. Through screening of more than 1.5 x 10(4) E. coli JM109 transformants, whose hlyB and hlyD genes had been mutagenized by error-prone PCR, we succeeded in isolating two mutants that had 27- and 15-fold-higher levels of subtilisin E secretion activity than the wild type did at 23 degrees C. These mutants also exhibited increased activity levels for secretion of a single-chain antibody-HlyA(218) fusion protein at 23 and 30 degrees C but unexpectedly not at 37 degrees C, suggesting that this improvement seems to be dependent on low temperature. One mutant (AE104) was found to have seven point mutations in both HlyB and HlyD, and an L448F substitution in HlyB was responsible for the improved secretion activity. Another mutant (AE129) underwent a single amino acid substitution (G654S) in HlyB. Secretion of c-Myc-HlyA(218) was detected only in the L448F mutant (AE104F) at 23 degrees C, whereas no secretion was observed in the wild type at any temperature. Furthermore, for the PTEN-HlyA(218) fusion protein, AE104F showed a 10-fold-higher level of secretion activity than the wild type did at 37 degrees C. This result indicates that the improved secretion activity of AE104F is not always dependent on low temperature.
Figures
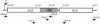





Similar articles
-
Mutations in HlyD, part of the type 1 translocator for hemolysin secretion, affect the folding of the secreted toxin.J Bacteriol. 2005 Nov;187(21):7471-80. doi: 10.1128/JB.187.21.7471-7480.2005. J Bacteriol. 2005. PMID: 16237030 Free PMC article.
-
Identification and preliminary characterization of temperature-sensitive mutations affecting HlyB, the translocator required for the secretion of haemolysin (HlyA) from Escherichia coli.Mol Gen Genet. 1994 Nov 15;245(4):431-40. doi: 10.1007/BF00302255. Mol Gen Genet. 1994. PMID: 7808392
-
Antibody analysis of the localisation, expression and stability of HlyD, the MFP component of the E. coli haemolysin translocator.Mol Gen Genet. 1999 Feb;261(1):122-32. doi: 10.1007/s004380050949. Mol Gen Genet. 1999. PMID: 10071218
-
E. coli hemolysin interactions with prokaryotic and eukaryotic cell membranes.Bioessays. 1992 Aug;14(8):519-25. doi: 10.1002/bies.950140804. Bioessays. 1992. PMID: 1365905 Review.
-
A molecular understanding of the catalytic cycle of the nucleotide-binding domain of the ABC transporter HlyB.Biochem Soc Trans. 2005 Nov;33(Pt 5):990-5. doi: 10.1042/BST20050990. Biochem Soc Trans. 2005. PMID: 16246029 Review.
Cited by
-
A novel genetic system for recombinant protein secretion in the Antarctic Pseudoalteromonas haloplanktis TAC125.Microb Cell Fact. 2006 Dec 14;5:40. doi: 10.1186/1475-2859-5-40. Microb Cell Fact. 2006. PMID: 17169153 Free PMC article.
-
Extracellular overexpression of recombinant Thermobifida fusca cutinase by alpha-hemolysin secretion system in E. coli BL21(DE3).Microb Cell Fact. 2012 Jan 12;11:8. doi: 10.1186/1475-2859-11-8. Microb Cell Fact. 2012. PMID: 22239833 Free PMC article.
-
Construction of leaky strains and extracellular production of exogenous proteins in recombinant Escherichia coli.Microb Biotechnol. 2014 Jul;7(4):360-70. doi: 10.1111/1751-7915.12127. Epub 2014 Apr 30. Microb Biotechnol. 2014. PMID: 24779863 Free PMC article.
-
An effective extracellular protein secretion by an ABC transporter system in Escherichia coli: statistical modeling and optimization of cyclodextrin glucanotransferase secretory production.J Ind Microbiol Biotechnol. 2011 Sep;38(9):1587-97. doi: 10.1007/s10295-011-0949-0. Epub 2011 Feb 19. J Ind Microbiol Biotechnol. 2011. PMID: 21336875
-
Genome engineering for improved recombinant protein expression in Escherichia coli.Microb Cell Fact. 2014 Dec 19;13:177. doi: 10.1186/s12934-014-0177-1. Microb Cell Fact. 2014. PMID: 25523647 Free PMC article. Review.
References
-
- Balakrishnan, L., C. Hughes, and V. Koronakis. 2001. Substrate-triggered recruitment of the TolC channel-tunnel during type I export of hemolysin by Escherichia coli. J. Mol. Biol. 313:501-510. - PubMed
-
- Benabdelhak, H., S. Kiontke, C. Horn, R. Ernst, M. A. Blight, I. B. Holland, and L. Schmitt. 2003. A specific interaction between the NBD of the ABC-transporter HlyB and a C-terminal fragment of its transport substrate haemolysin A. J. Mol. Biol. 327:1169-1179. - PubMed
-
- Blight, M. A., and I. B. Holland. 1990. Structure and function of haemolysin B, P-glycoprotein and other members of a novel family of membrane translocators. Mol. Microbiol. 4:873-880. - PubMed
-
- Blight, M. A., C. Chervaux, and I. B. Holland. 1994. Protein secretion pathway in Escherichia coli. Curr. Opin. Biotechnol. 5:468-474. - PubMed
-
- Blight, M. A., and I. B. Holland. 1994. Heterologous protein secretion and the versatile Escherichia coli haemolysin translocator. Trends Biotechnol. 12:450-455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

