Tetracycline-dependent conditional gene knockout in Bacillus subtilis
- PMID: 15691923
- PMCID: PMC546824
- DOI: 10.1128/AEM.71.2.728-733.2005
Tetracycline-dependent conditional gene knockout in Bacillus subtilis
Abstract
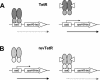
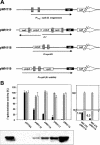
Reversible tetracycline-dependent gene regulation allows induction of expression with the tetracycline repressor (TetR) or gene silencing with the newly developed reverse mutant revTetR. We report here the implementation of both approaches with full regulatory range in gram-positive bacteria as exemplified in Bacillus subtilis. A chromosomally located gene is controlled by one or two tet operators. The precise adjustment of regulatory windows is accomplished by adjusting tetR or revtetR expression via different promoters. The most efficient induction was 300-fold in the presence of 0.4 microM anhydrotetracycline obtained with a Pr-xylA-tetR fusion. Reversible 500-fold gene knockouts were obtained in B. subtilis after adjusting expression of revTetR by synthetically designed promoters. We anticipate that these tools will also be useful in many other gram-positive bacteria.
Figures
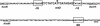
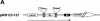
Similar articles
-
Integrative elements for Bacillus subtilis yielding tetracycline-dependent growth phenotypes.Nucleic Acids Res. 2005 Oct 12;33(18):e153. doi: 10.1093/nar/gni154. Nucleic Acids Res. 2005. PMID: 16221969 Free PMC article.
-
Two mutations in the tetracycline repressor change the inducer anhydrotetracycline to a corepressor.Nucleic Acids Res. 2004 Feb 4;32(2):842-7. doi: 10.1093/nar/gkh200. Print 2004. Nucleic Acids Res. 2004. PMID: 14764926 Free PMC article.
-
Transcription of the xyl operon is controlled in Bacillus subtilis by tandem overlapping operators spaced by four base-pairs.J Mol Biol. 1994 Oct 28;243(3):413-24. doi: 10.1006/jmbi.1994.1669. J Mol Biol. 1994. PMID: 7966270
-
Functions of tetracycline efflux proteins that do not involve tetracycline.J Mol Microbiol Biotechnol. 2001 Apr;3(2):237-46. J Mol Microbiol Biotechnol. 2001. PMID: 11321579 Review.
-
Mechanisms underlying expression of Tn10 encoded tetracycline resistance.Annu Rev Microbiol. 1994;48:345-69. doi: 10.1146/annurev.mi.48.100194.002021. Annu Rev Microbiol. 1994. PMID: 7826010 Review.
Cited by
-
New methods for tightly regulated gene expression and highly efficient chromosomal integration of cloned genes for Methanosarcina species.Archaea. 2008 Dec;2(3):193-203. doi: 10.1155/2008/534081. Archaea. 2008. PMID: 19054746 Free PMC article.
-
Recombinant Mycobacterium bovis BCG as an HIV vaccine vector.Curr HIV Res. 2010 Jun;8(4):282-98. doi: 10.2174/157016210791208686. Curr HIV Res. 2010. PMID: 20353397 Free PMC article. Review.
-
Integrative elements for Bacillus subtilis yielding tetracycline-dependent growth phenotypes.Nucleic Acids Res. 2005 Oct 12;33(18):e153. doi: 10.1093/nar/gni154. Nucleic Acids Res. 2005. PMID: 16221969 Free PMC article.
-
The application of Tet repressor in prokaryotic gene regulation and expression.Microb Biotechnol. 2008 Jan;1(1):2-16. doi: 10.1111/j.1751-7915.2007.00001.x. Microb Biotechnol. 2008. PMID: 21261817 Free PMC article. Review.
-
Staphylococcus aureus TargetArray: comprehensive differential essential gene expression as a mechanistic tool to profile antibacterials.Antimicrob Agents Chemother. 2010 Sep;54(9):3659-70. doi: 10.1128/AAC.00308-10. Epub 2010 Jun 14. Antimicrob Agents Chemother. 2010. PMID: 20547796 Free PMC article.
References
-
- Arantes, O., and D. Lereclus. 1991.Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115-119. - PubMed
-
- Fukiya, S., H. Mizoguchi, and H. Mori. 2004. An improved method for deleting large regions of Escherichia coli K-12 chromosome using a combination of Cre/loxP and lambda Red. FEMS Microbiol. Lett. 234:325-331. - PubMed
-
- Geissendörfer, M., and W. Hillen. 1990. Regulated expression of heterologous genes in Bacillus subtilis using the Tn10 encoded tet regulatory elements. Appl. Microbiol. Biotechnol. 33:657-663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

