Role of the bga1-encoded extracellular {beta}-galactosidase of Hypocrea jecorina in cellulase induction by lactose
- PMID: 15691940
- PMCID: PMC546727
- DOI: 10.1128/AEM.71.2.851-857.2005
Role of the bga1-encoded extracellular {beta}-galactosidase of Hypocrea jecorina in cellulase induction by lactose
Abstract
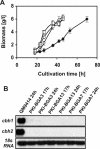
Lactose is the only soluble and economically feasible carbon source for the production of cellulases or heterologous proteins regulated by cellulase expression signals by Hypocrea jecorina (Trichoderma reesei). We investigated the role of the major beta-galactosidase of H. jecorina in lactose metabolism and cellulase induction. A genomic copy of the bga1 gene was cloned, and this copy encodes a 1,023-amino-acid protein with a 20-amino-acid signal sequence. This protein has a molecular mass of 109.3 kDa, belongs to glycosyl hydrolase family 35, and is the major extracellular beta-galactosidase during growth on lactose. Its transcript was abundant during growth on l-arabinose and l-arabinitol but was much less common when the organism was grown on lactose, d-galactose, galactitol, d-xylose, and xylitol. Deltabga1 strains grow more slowly and accumulate less biomass on lactose, but the cellobiohydrolase I and II gene expression and the final cellulase yields were comparable to those of the parental strain. Overexpression of bga1 under the control of the pyruvate kinase promoter reduced the lag phase, increased growth on lactose, and limited transcription of cellobiohydrolases. We detected an additional extracellular beta-galactosidase activity that was not encoded by bga1 but no intracellular beta-galactosidase activity. In conclusion, cellulase production on lactose occurs when beta-galactosidase activity levels are low but decreases as the beta-galactosidase activities increase. The data indicate that bga1-encoded beta-galactosidase activity is a critical factor for cellulase production on lactose.
Figures
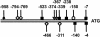


References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Stuhl. 2003. Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
-
- Bannai, H., Y. Tamada, O. Maruyama, K. Nakai, and S. Miyano. 2002. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 18:298-305. - PubMed
-
- Berka, R. M., J. A. Hucul, and M. Ward. April 1998. Increased production of β-galactosidase in Aspergillus oryzae. U.S. patent 5736374-A 2.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

