High motility reduces grazing mortality of planktonic bacteria
- PMID: 15691949
- PMCID: PMC546711
- DOI: 10.1128/AEM.71.2.921-929.2005
High motility reduces grazing mortality of planktonic bacteria
Abstract
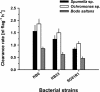
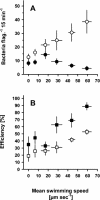
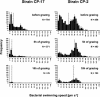
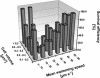
We tested the impact of bacterial swimming speed on the survival of planktonic bacteria in the presence of protozoan grazers. Grazing experiments with three common bacterivorous nanoflagellates revealed low clearance rates for highly motile bacteria. High-resolution video microscopy demonstrated that the number of predator-prey contacts increased with bacterial swimming speed, but ingestion rates dropped at speeds of >25 microm s(-1) as a result of handling problems with highly motile cells. Comparative studies of a moderately motile strain (<25 microm s(-1)) and a highly motile strain (>45 microm s(-1)) further revealed changes in the bacterial swimming speed distribution due to speed-selective flagellate grazing. Better long-term survival of the highly motile strain was indicated by fourfold-higher bacterial numbers in the presence of grazing compared to the moderately motile strain. Putative constraints of maintaining high swimming speeds were tested at high growth rates and under starvation with the following results: (i) for two out of three strains increased growth rate resulted in larger and slower bacterial cells, and (ii) starved cells became smaller but maintained their swimming speeds. Combined data sets for bacterial swimming speed and cell size revealed highest grazing losses for moderately motile bacteria with a cell size between 0.2 and 0.4 microm(3). Grazing mortality was lowest for cells of >0.5 microm(3) and small, highly motile bacteria. Survival efficiencies of >95% for the ultramicrobacterial isolate CP-1 (< or =0.1 microm(3), >50 microm s(-1)) illustrated the combined protective action of small cell size and high motility. Our findings suggest that motility has an important adaptive function in the survival of planktonic bacteria during protozoan grazing.
Figures


References
-
- Beck, K. 2000. Experimentelle Überprüfung der “Intermediate disturbance hypothesis” (Connell 1978) an Modell-Lebensgemeinschaften planktischer Bakterien. Ph.D. dissertation. University of Kiel, Kiel, Germany.
-
- Blackburn, N., T. Fenchel, and J. G. Mitchell. 1998. Microscale nutrient patches in planktonic habitats shown by chemotactic bacteria. Science 282:2254-2256. - PubMed
-
- Boenigk, J., C. Matz, K. Jürgens, and H. Arndt. 2001. The influence of preculture conditions and food quality on the ingestion and digestion process of three species of heterotrophic nanoflagellates. Microb. Ecol. 42:168-176. - PubMed
-
- Chrzanowski, T. H., and K. Šimek. 1990. Prey-size selection by freshwater flagellated protozoa. Limnol. Oceanogr. 35:1429-1436.
-
- del Giorgio, P. A., J. M. Gasol, D. Vaque, P. Mura, S. Agusti, and C. M. Duarte. 1996. Bacterioplankton community structure—protists control net production and the proportion of active bacteria in a coastal marine community. Limnol. Oceanogr. 41:1169-1179.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

