Bacterial degradation of cyanide and its metal complexes under alkaline conditions
- PMID: 15691951
- PMCID: PMC546731
- DOI: 10.1128/AEM.71.2.940-947.2005
Bacterial degradation of cyanide and its metal complexes under alkaline conditions
Abstract
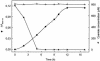
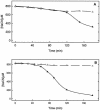
A bacterial strain able to use cyanide as the sole nitrogen source under alkaline conditions has been isolated. The bacterium was classified as Pseudomonas pseudoalcaligenes by comparison of its 16S RNA gene sequence to those of existing strains and deposited in the Coleccion Espanola de Cultivos Tipo (Spanish Type Culture Collection) as strain CECT5344. Cyanide consumption is an assimilative process, since (i) bacterial growth was concomitant and proportional to cyanide degradation and (ii) the bacterium stoichiometrically converted cyanide into ammonium in the presence of l-methionine-d,l-sulfoximine, a glutamine synthetase inhibitor. The bacterium was able to grow in alkaline media, up to an initial pH of 11.5, and tolerated free cyanide in concentrations of up to 30 mM, which makes it a good candidate for the biological treatment of cyanide-contaminated residues. Both acetate and d,l-malate were suitable carbon sources for cyanotrophic growth, but no growth was detected in media with cyanide as the sole carbon source. In addition to cyanide, P. pseudoalcaligenes CECT5344 used other nitrogen sources, namely ammonium, nitrate, cyanate, cyanoacetamide, nitroferricyanide (nitroprusside), and a variety of cyanide-metal complexes. Cyanide and ammonium were assimilated simultaneously, whereas cyanide strongly inhibited nitrate and nitrite assimilation. Cyanase activity was induced during growth with cyanide or cyanate, but not with ammonium or nitrate as the nitrogen source. This result suggests that cyanate could be an intermediate in the cyanide degradation pathway, but alternative routes cannot be excluded.
Figures


Similar articles
-
Alkaline cyanide biodegradation by Pseudomonas pseudoalcaligenes CECT5344.Biochem Soc Trans. 2005 Feb;33(Pt 1):168-9. doi: 10.1042/BST0330168. Biochem Soc Trans. 2005. PMID: 15667296
-
Characterization of the Pseudomonas pseudoalcaligenes CECT5344 Cyanase, an enzyme that is not essential for cyanide assimilation.Appl Environ Microbiol. 2008 Oct;74(20):6280-8. doi: 10.1128/AEM.00916-08. Epub 2008 Aug 15. Appl Environ Microbiol. 2008. PMID: 18708510 Free PMC article.
-
Cyanate Assimilation by the Alkaliphilic Cyanide-Degrading Bacterium Pseudomonas pseudoalcaligenes CECT5344: Mutational Analysis of the cyn Gene Cluster.Int J Mol Sci. 2019 Jun 20;20(12):3008. doi: 10.3390/ijms20123008. Int J Mol Sci. 2019. PMID: 31226739 Free PMC article.
-
Bacterial cyanide degradation is under review: Pseudomonas pseudoalcaligenes CECT5344, a case of an alkaliphilic cyanotroph.Biochem Soc Trans. 2011 Jan;39(1):269-74. doi: 10.1042/BST0390269. Biochem Soc Trans. 2011. PMID: 21265786 Review.
-
Cyanide metabolism of Pseudomonas pseudoalcaligenes CECT5344: role of siderophores.Biochem Soc Trans. 2006 Feb;34(Pt 1):152-5. doi: 10.1042/BST0340152. Biochem Soc Trans. 2006. PMID: 16417508 Review.
Cited by
-
Spectral characterization of a pteridine derivative from cyanide-utilizing bacterium Bacillus subtilis - JN989651.J Microbiol. 2015 Apr;53(4):262-71. doi: 10.1007/s12275-015-4138-0. Epub 2015 Mar 4. J Microbiol. 2015. PMID: 25740375
-
A Case of Adaptive Laboratory Evolution (ALE): Biodegradation of Furfural by Pseudomonas pseudoalcaligenes CECT 5344.Genes (Basel). 2019 Jun 29;10(7):499. doi: 10.3390/genes10070499. Genes (Basel). 2019. PMID: 31261932 Free PMC article.
-
Free cyanide and thiocyanate biodegradation by Pseudomonas aeruginosa STK 03 capable of heterotrophic nitrification under alkaline conditions.3 Biotech. 2016 Jun;6(1):6. doi: 10.1007/s13205-015-0317-2. Epub 2015 Dec 31. 3 Biotech. 2016. PMID: 28330076 Free PMC article.
-
A Cyanide-Induced 3-Cyanoalanine Nitrilase in the Cyanide-Assimilating Bacterium Pseudomonas pseudoalcaligenes Strain CECT 5344.Appl Environ Microbiol. 2017 Apr 17;83(9):e00089-17. doi: 10.1128/AEM.00089-17. Print 2017 May 1. Appl Environ Microbiol. 2017. PMID: 28235872 Free PMC article.
-
Essential role of cytochrome bd-related oxidase in cyanide resistance of Pseudomonas pseudoalcaligenes CECT5344.Appl Environ Microbiol. 2007 Aug;73(16):5118-24. doi: 10.1128/AEM.00503-07. Epub 2007 Jun 15. Appl Environ Microbiol. 2007. PMID: 17574992 Free PMC article.
References
-
- Adjei, M. D., and Y. Ohta. 1999. Isolation and characterization of a cyanide-utilizing Burkholderia cepacia strain. World J. Microbiol. Biotechnol. 15:699-704.
-
- Adjei, M. D., and Y. Ohta. 2000. Factors affecting the biodegradation of cyanide by Burkholderia cepacia strain C-3. J. Biosci. Bioeng. 3:274-277. - PubMed
-
- Anderson, P. M. 1980. Purification and properties of the inducible enzyme cyanase. Biochemistry 19:2882-2888. - PubMed
-
- Andrews, S. C., Robinson, A. K., and F. Rodríguez-Quiñones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. - PubMed
-
- Asmus, E., and H. Garschagen. 1953. The use of barbituric acid for the photometric determination of cyanide and thiocyanate. Z. Anal. Chem. 138:414-422.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

