Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni
- PMID: 15691957
- PMCID: PMC546671
- DOI: 10.1128/AEM.71.2.987-992.2005
Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni
Abstract
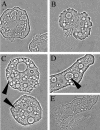
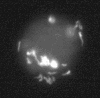
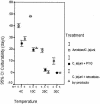
We showed by a laboratory experiment that four different Campylobacter jejuni strains are able to infect the protozoan Acanthamoeba polyphaga. C. jejuni cells survived for longer periods when cocultured with amoebae than when grown in culture alone. The infecting C. jejuni cells aggregated in amoebic vacuoles, in which they were seen to be actively moving. Furthermore, a resuscitation of bacterial cultures that were previously negative in culturability tests was observed after reinoculation into fresh amoeba cultures. After spontaneous rupture of the amoebae, C. jejuni could be detected by microscopy and culturability tests. Our results indicate that amoebae may serve as a nonvertebrate reservoir for C. jejuni in the environment.
Figures

Similar articles
-
Fate of internalized Campylobacter jejuni and Mycobacterium avium from encysted and excysted Acanthamoeba polyphaga.Exp Parasitol. 2019 Apr;199:104-110. doi: 10.1016/j.exppara.2019.03.011. Epub 2019 Mar 19. Exp Parasitol. 2019. PMID: 30902623
-
Colonization of broilers by Campylobacter jejuni internalized within Acanthamoeba castellanii.Arch Microbiol. 2008 Feb;189(2):175-9. doi: 10.1007/s00203-007-0303-0. Epub 2007 Sep 19. Arch Microbiol. 2008. PMID: 17882400
-
Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model.Int J Food Microbiol. 2006 Mar 1;107(1):83-91. doi: 10.1016/j.ijfoodmicro.2005.08.015. Epub 2005 Nov 14. Int J Food Microbiol. 2006. PMID: 16290304
-
Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms.Int J Med Microbiol. 2010 Apr;300(4):205-11. doi: 10.1016/j.ijmm.2009.07.002. Epub 2009 Aug 8. Int J Med Microbiol. 2010. PMID: 19665925 Review.
-
A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut.Crit Rev Microbiol. 2012 Feb;38(1):17-29. doi: 10.3109/1040841X.2011.615298. Epub 2011 Oct 13. Crit Rev Microbiol. 2012. PMID: 21995731 Review.
Cited by
-
Transient internalization of Campylobacter jejuni in Amoebae enhances subsequent invasion of human cells.Microbiology (Reading). 2022 Feb;168(2):001143. doi: 10.1099/mic.0.001143. Microbiology (Reading). 2022. PMID: 35175913 Free PMC article.
-
Persistence of free-living protozoan communities across rearing cycles in commercial poultry houses.Appl Environ Microbiol. 2011 Mar;77(5):1763-9. doi: 10.1128/AEM.01756-10. Epub 2011 Jan 14. Appl Environ Microbiol. 2011. PMID: 21239551 Free PMC article.
-
Giant viruses, giant chimeras: the multiple evolutionary histories of Mimivirus genes.BMC Evol Biol. 2008 Jan 18;8:12. doi: 10.1186/1471-2148-8-12. BMC Evol Biol. 2008. PMID: 18205905 Free PMC article.
-
Detection of Campylobacter spp. in water by dead-end ultrafiltration and application at farm level.J Appl Microbiol. 2019 Oct;127(4):1270-1279. doi: 10.1111/jam.14379. Epub 2019 Jul 22. J Appl Microbiol. 2019. PMID: 31291690 Free PMC article.
-
Detection of Vibrio cholerae and Acanthamoeba species from same natural water samples collected from different cholera endemic areas in Sudan.BMC Res Notes. 2011 Apr 7;4:109. doi: 10.1186/1756-0500-4-109. BMC Res Notes. 2011. PMID: 21470437 Free PMC article.
References
-
- Ahearn, D. G., and M. M. Gabriel. 1997. Contact lenses, disinfectants and acanthamoebae keratitis. Adv. Appl. Microbiol. 43:35-56. - PubMed
-
- Barker, J., and R. W. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. - PubMed
-
- Buswell, C. M., Y. M. Herlihy, L. M. Lawrence, J. T. M. McGuiggan, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733-741. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

