Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1-/- mice
- PMID: 15692571
- PMCID: PMC549615
- DOI: 10.1038/sj.emboj.7600542
Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1-/- mice
Abstract
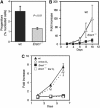
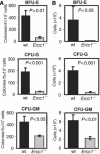
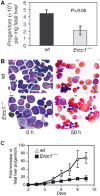
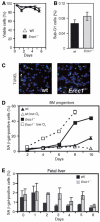
The ERCC1-XPF heterodimer is a structure-specific endonuclease involved in both nucleotide excision repair and interstrand crosslink repair. Mice carrying a genetic defect in Ercc1 display symptoms suggestive of a progressive, segmental progeria, indicating that disruption of one or both of these DNA damage repair pathways accelerates aging. In the hematopoietic system, there are defined age-associated changes for which the cause is unknown. To determine if DNA repair is critical to prolonged hematopoietic function, hematopoiesis in Ercc1-/- mice was compared to that in young and old wild-type mice. Ercc1-/- mice (3-week-old) exhibited multilineage cytopenia and fatty replacement of bone marrow, similar to old wild-type mice. In addition, the proliferative reserves of hematopoietic progenitors and stress erythropoiesis were significantly reduced in Ercc1-/- mice compared to age-matched controls. These features were not seen in nucleotide excision repair-deficient Xpa-/- mice, but are characteristic of Fanconi anemia, a human cancer syndrome caused by defects in interstrand crosslink repair. These data support the hypothesis that spontaneous interstrand crosslink damage contributes to the functional decline of the hematopoietic system associated with aging.
Figures



References
-
- Alter BP, Knobloch ME, Weinberg RS (1991) Erythropoiesis in Fanconi's anemia. Blood 78: 602–608 - PubMed
-
- Bagnara GP, Strippoli P, Bonsi L, Brizzi MF, Avanzi GC, Timeus F, Ramenghi U, Piaggio G, Tong J, Podesta M, Paolucci G, Pegoraro L, Gabutti V, Bacigalupo A (1992) Effect of stem cell factor on colony growth from acquired and constitutional (Fanconi) aplastic anemia. Blood 80: 382–387 - PubMed
-
- Balducci L, Extermann M (1997) Cancer chemotherapy in the older patient: what the medical oncologist needs to know. Cancer 80: 1317–1322 - PubMed
-
- Barja G (2002) Rate of generation of oxidative stress-related damage and animal longevity. Free Radic Biol Med 33: 1167–1172 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

