The Drosophila mitochondrial ribosomal protein mRpL12 is required for Cyclin D/Cdk4-driven growth
- PMID: 15692573
- PMCID: PMC548645
- DOI: 10.1038/sj.emboj.7600523
The Drosophila mitochondrial ribosomal protein mRpL12 is required for Cyclin D/Cdk4-driven growth
Abstract
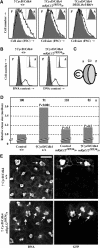
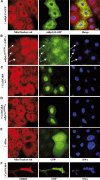
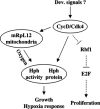
The Drosophila melanogaster cyclin-dependent protein kinase complex CycD/Cdk4 stimulates both cell cycle progression and cell growth (accumulation of mass). CycD/Cdk4 promotes cell cycle progression via the well-characterized RBF/E2F pathway, but our understanding of how growth is stimulated is still limited. To identify growth regulatory targets of CycD/Cdk4, we performed a loss-of-function screen for modifiers of CycD/Cdk4-induced overgrowth of the Drosophila eye. One mutation that suppressed CycD/Cdk4 was in a gene encoding the mitochondrial ribosomal protein, mRpL12. We show here that mRpL12 is required for CycD/Cdk4-induced cell growth. Cells homozygous mutant for mRpL12 have reduced mitochondrial activity, and exhibit growth defects that are very similar to those of cdk4 null cells. CycD/Cdk4 stimulates mitochondrial activity, and this is mRpL12 dependent. Hif-1 prolyl hydroxylase (Hph), another effector of CycD/Cdk4, regulates growth and is required for inhibition of the hypoxia-inducible transcription factor 1 (Hif-1). Both functions depend on mRpL12 dosage, suggesting that CycD/Cdk4, mRpL12 and Hph function together in a common pathway that controls cell growth via affecting mitochondrial activity.
Figures




Similar articles
-
Drosophila cyclin D/Cdk4 requires Hif-1 prolyl hydroxylase to drive cell growth.Dev Cell. 2004 Feb;6(2):241-51. doi: 10.1016/s1534-5807(03)00409-x. Dev Cell. 2004. PMID: 14960278
-
Cyclin D/Cdk4: new insights from Drosophila.Cell Cycle. 2004 May;3(5):558-60. Epub 2004 May 19. Cell Cycle. 2004. PMID: 15044855
-
The Drosophila cyclin D-Cdk4 complex promotes cellular growth.EMBO J. 2000 Sep 1;19(17):4543-54. doi: 10.1093/emboj/19.17.4543. EMBO J. 2000. PMID: 10970848 Free PMC article.
-
A genetic modifier screen identifies multiple genes that interact with Drosophila Rap/Fzr and suggests novel cellular roles.J Neurogenet. 2007 Jul-Sep;21(3):105-51. doi: 10.1080/01677060701503140. J Neurogenet. 2007. PMID: 17849284 Review.
-
Growth regulation by oncogenes--new insights from model organisms.Curr Opin Genet Dev. 2001 Feb;11(1):19-26. doi: 10.1016/s0959-437x(00)00151-9. Curr Opin Genet Dev. 2001. PMID: 11163146 Review.
Cited by
-
Ribosomal protein L10 in mitochondria serves as a regulator for ROS level in pancreatic cancer cells.Redox Biol. 2018 Oct;19:158-165. doi: 10.1016/j.redox.2018.08.016. Epub 2018 Aug 24. Redox Biol. 2018. PMID: 30172100 Free PMC article.
-
Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function.Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11567-72. doi: 10.1073/pnas.0603363103. Epub 2006 Jul 24. Proc Natl Acad Sci U S A. 2006. PMID: 16864783 Free PMC article.
-
Involvement of the mitochondrial protein translocator component tim50 in growth, cell proliferation and the modulation of respiration in Drosophila.Genetics. 2007 Jun;176(2):927-36. doi: 10.1534/genetics.107.072074. Epub 2007 Apr 15. Genetics. 2007. PMID: 17435247 Free PMC article.
-
SQSTM1/p62 Controls mtDNA Expression and Participates in Mitochondrial Energetic Adaption via MRPL12.iScience. 2020 Aug 21;23(8):101428. doi: 10.1016/j.isci.2020.101428. Epub 2020 Aug 1. iScience. 2020. PMID: 32805647 Free PMC article.
-
A genetic screen for dominant modifiers of a small-wing phenotype in Drosophila melanogaster identifies proteins involved in splicing and translation.Genetics. 2005 Oct;171(2):597-614. doi: 10.1534/genetics.105.045021. Epub 2005 Jul 5. Genetics. 2005. PMID: 15998720 Free PMC article.
References
-
- Agani FH, Pichiule P, Carlos Chavez J, LaManna JC (2002) Inhibitors of mitochondrial complex I attenuate the accumulation of hypoxia-inducible factor-1 during hypoxia in Hep3B cells. Comp Biochem Physiol A 132: 107–109 - PubMed
-
- Agani FH, Pichiule P, Chavez JC, LaManna JC (2000) The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem 275: 35863–35867 - PubMed
-
- Braun L, Kardon T, Reisz-Porszasz ZS, Banhegyi G, Mandl J (2001) The regulation of the induction of vascular endothelial growth factor at the onset of diabetes in spontaneously diabetic rats. Life Sci 69: 2533–2542 - PubMed
-
- Britton JS, Edgar BA (1998) Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development 125: 2149–2158 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

