Dendritic cells derived from metastatic cancer patients vaccinated with allogeneic dendritic cell-autologous tumor cell hybrids express more CD86 and induce higher levels of interferon-gamma in mixed lymphocyte reactions
- PMID: 15693140
- PMCID: PMC11034268
- DOI: 10.1007/s00262-004-0550-8
Dendritic cells derived from metastatic cancer patients vaccinated with allogeneic dendritic cell-autologous tumor cell hybrids express more CD86 and induce higher levels of interferon-gamma in mixed lymphocyte reactions
Abstract
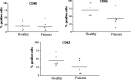
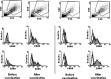
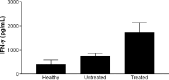
Dendritic cells (DCs) are highly effective antigen-presenting cells that, when derived from cancer patients, seem to be functionally deficient. Herein, we show that vaccination with allogeneic DC-autologous tumor cell hybrids affects the phenotype and improves the function of monocyte-derived DCs (Mo-DCs) from cancer patients. Mononuclear cells were isolated from patients' peripheral blood by density gradient centrifugation, and adherent cells were cultured in medium containing GM-CSF plus IL-4 and, after 5 days, TNF-alpha. After 2 more days, Mo-DCs were harvested and their CD80, CD86, and CD83 expression was assessed by flow cytometry. They were also used as stimulators in mixed lymphocyte reactions (MLR), where IFN-gamma production was measured by ELISA. Mo-DCs from unvaccinated patients expressed significantly lower levels of CD86, and tended to express lower levels of CD83 than Mo-DCs from healthy donors. However, Mo-DCs generated after hybrid cell vaccination presented increased expression of the same markers and induced significantly higher levels of IFN-gamma in MLR. These results indicate that the use of allogeneic DC-based cancer vaccines induces recovery of DC function in metastatic cancer patients and, therefore, could precede the use of autologous DCs for vaccine preparation. Such an approach could be relevant and should be investigated in clinical trials.
Figures

Similar articles
-
Low levels of interferon-alpha induce CD86 (B7.2) expression and accelerates dendritic cell maturation from human peripheral blood mononuclear cells.Scand J Immunol. 1999 Nov;50(5):499-509. doi: 10.1046/j.1365-3083.1999.00625.x. Scand J Immunol. 1999. PMID: 10564553
-
Decreased inducible expression of CD80 and CD86 in human monocytes after ultraviolet-B irradiation: its involvement in inactivation of allogenecity.Blood. 1996 Mar 15;87(6):2386-93. Blood. 1996. PMID: 8630402
-
Replicative response, immunophenotype, and functional activity of monocyte-derived versus CD34(+)-derived dendritic cells following exposure to various expansion and maturational stimuli.Clin Immunol. 2001 Feb;98(2):280-92. doi: 10.1006/clim.2000.4968. Clin Immunol. 2001. PMID: 11161986
-
Novel dendritic cell-based vaccination in late stage melanoma.Hum Vaccin Immunother. 2014;10(11):3132-8. doi: 10.4161/hv.29110. Hum Vaccin Immunother. 2014. PMID: 25483650 Free PMC article. Review.
-
Cell-based vaccines for renal cell carcinoma: genetically-engineered tumor cells and monocyte-derived dendritic cells.World J Urol. 2005 Jul;23(3):166-74. doi: 10.1007/s00345-005-0505-5. Epub 2005 Jul 5. World J Urol. 2005. PMID: 15997395 Review.
Cited by
-
High frequency of immature dendritic cells and altered in situ production of interleukin-4 and tumor necrosis factor-alpha in lung cancer.Cancer Immunol Immunother. 2008 Sep;57(9):1335-45. doi: 10.1007/s00262-008-0468-7. Epub 2008 Feb 20. Cancer Immunol Immunother. 2008. PMID: 18286287 Free PMC article.
-
Human papillomavirus infection affects the immune microenvironment and antigen presentation in penile cancer.Front Oncol. 2024 Oct 18;14:1463445. doi: 10.3389/fonc.2024.1463445. eCollection 2024. Front Oncol. 2024. PMID: 39493451 Free PMC article.
-
Antigen-specific polyclonal cytotoxic T lymphocytes induced by fusions of dendritic cells and tumor cells.J Biomed Biotechnol. 2010;2010:752381. doi: 10.1155/2010/752381. Epub 2010 Apr 7. J Biomed Biotechnol. 2010. PMID: 20379390 Free PMC article.
-
Therapeutic dendritic cell vaccine preparation using tumor RNA transfection: a promising approach for the treatment of prostate cancer.Genet Vaccines Ther. 2008 Jan 18;6:2. doi: 10.1186/1479-0556-6-2. Genet Vaccines Ther. 2008. PMID: 18205933 Free PMC article.
-
Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines.Cancer Immunol Immunother. 2008 Oct;57(10):1569-77. doi: 10.1007/s00262-008-0536-z. Epub 2008 Jun 4. Cancer Immunol Immunother. 2008. PMID: 18523771 Free PMC article. Review.
References
-
- Barbuto JAM, Ensina LF, Neves AR, Bergami-Santos PC, Marques R, Leite KRM, Camara-Lopes LH, Buzaid AC (2002) Dendritic-tumor hybrid cell therapeutic vaccination for metastatic melanoma and renal cell carcinoma patients. International symposium on predictive oncology and intervention strategies, Paris, France, 9--12 February 2002. http://www.cancerprev.org/Journal/Issues/26/101/1011/4579 Cited 24 August 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

