Valdecoxib for treatment of primary dysmenorrhea. A randomized, double-blind comparison with placebo and naproxen
- PMID: 15693930
- PMCID: PMC1490036
- DOI: 10.1111/j.1525-1497.2004.30052.x
Valdecoxib for treatment of primary dysmenorrhea. A randomized, double-blind comparison with placebo and naproxen
Abstract
Objective: To compare the analgesic efficacy of valdecoxib with placebo and naproxen sodium for relieving menstrual cramping and pain due to primary dysmenorrhea.
Design: Single-center, double-blind study with a 4-period, 4-sequence crossover design. Patients assessed pain intensity and pain relief at regular intervals up to 12 hours following the initial dose.
Setting: Privately owned outpatient clinic.
Patients/participants: One hundred twenty patients with moderate to severe menstrual cramping were randomized. Eighty-seven patients completed all treatment cycles.
Interventions: Valdecoxib 20 mg or 40 mg, naproxen sodium 550 mg, or placebo twice a day as required for < or =3 days in a single menstrual cycle.
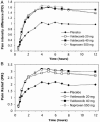
Measurements and main results: Both doses of valdecoxib (20 and 40 mg) were comparable to naproxen sodium and superior to placebo at all time points assessed for each of the primary end points. Valdecoxib and naproxen sodium had comparable onset and duration of action. Although the study design allowed patients 2 doses per day, only 15% and 20% of patients in the valdecoxib 20 mg and valdecoxib 40 mg groups, respectively, required remedication within the first 12 hours. The incidence of adverse events was similar between active and placebo groups.
Conclusion: Valdecoxib provided a fast onset of analgesic action, a level of efficacy similar to naproxen sodium, and a high level of patient satisfaction in the relief of menstrual pain due to primary dysmenorrhea. Valdecoxib was effective and well tolerated and thus appears to be a viable treatment for menstrual pain due to primary dysmenorrhea.
Figures

References
-
- Dawood MY. Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. Am J Med. 1988;84(suppl 5A):23–9. - PubMed
-
- Rosenwaks Z, Seegar-Jones G. Menstrual pain: its origin and pathogenesis. J Reprod Med. 1980;25(4 suppl):207–12. - PubMed
-
- García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Erratum in Lancet. 1994;343:1048. 1994;343:769–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
